In-depth characterization of proteins, antibodies, and peptides...
Biologically derived products such as recombinant proteins, peptides, and antibodies (e.g. Monoclonal Antibody Drug Conjugates (ADC)) are complex systems which are influenced by post-translational modifications (PTMs), oligomerization states, and formulations. In order to assess the overall effect of these modifications, a robust biophysical and physicochemical program needs to be implemented. Assessment from the primary structure (amino acid sequencing), PTMs (phosphorylation, glycosylation, acetylation, oxidation, etc.), secondary structure (unfolding vs folded), and overall tertiary structure (identification and quantitation of Higher Order Structures (HOS)) are just some of the crucial characterization steps required.A complete biophysical characterization can address the structural differences of the biological product occurring during the manufacturing process that may result in significantly different properties that may affect efficacy, toxicity, immunogenicity, and stability.
The ICHQ6B Guidelines for: Test procedures and Acceptance Criteria of
Biotechnological/Biological Productsas well as the Guidelines for
Qualityset forth guidance for the analysis of proteins and peptides and are a good framework for developing strategies for comprehensive understanding of large molecules.
Stability
Comparability in Manufacturing
Specifications
The Quality Guidance offers information on the development of analytical methods for API control and consistency. The FDA offers similar guidance for developing and validating analytical methods for biologically derived therapeutics.6,7Triclinic Labs offers not only a comprehensive suite of techniques to provide characterization of biologic drug substances but also expertise in the analysis of data in order to direct guidance on moving your large molecule programs forward.
The Stability Guidance recommends tests for both chemical stability and biological activity of peptides and proteins.
The Comparability Guidance establishes criteria for biologics that are based on the understanding that “changes in a manufacturing process, equipment, or facilities may result in changes to a biological product that would affect the product’s safety and efficacy”. Comparability protocols are developed to reduce the risk of these changes by developing strategies for comparing the physiochemical and biological properties of these materials. These properties include stability, identity, purity and impurity, potency, aggregation state, structure, high order structure, and activity.
Generally, characterization of sequence, structure, and PTMs are undertaken for biophysical characterization. Evaluation of binding affinities to specific ligands can also be undertaken.
Ask Our Scientists!
Request More Info
CHARACTERIZATION LEVELS AND AVAILABLE TECHNIQUES
-
Primary Structure and Post-translational Modification (PTM) Analysis
Techniques available:
- Protease Profiling by Mass Spectrometry (MS)
In order to characterize the primary structure of proteins, antibodies and peptides, mass spectrometry will be employed. Protease profiling of the samples for example, using Trypsin, Lys C, Glu C, Asp N and Chymotrypsin will be performed to obtain the greatest amino acid coverage. During the course of these experiments, an initial analysis of post translational modifications (PTMs) will also be performed.
-
Secondary Structure Analysis
Techniques available:
-
Far/Near UV Circular Dichroism
Secondary and tertiary structure analyses will be performed by Circular Dichroism (CD). Near and Far CD experiments will provide information about any changes in the secondary structure (alpha, beta and random motifs) and the overall presence of higher order structures (HOS).
-
Fourier Transform Raman (FT-Raman)/ Fourier Transform Infrared (FTIR) Spectroscopy (Solid-State Analysis)
FT Raman/FTIR analysis of the lyophilized samples will be conducted to assess whether or not the lyophilization process has affected the overall chemical fingerprint and secondary structure of the samples. Changes in the internal microenvironment of the sample along with analysis of the Amide I, II and II regions can give insight into the formulation and/or lyophilization process. These experiments can give valuable information on the overall stability of the samples.
-
Far/Near UV Circular Dichroism
-
Tertiary Structure Analysis
Techniques available:
-
Analytical Ultracentrifugation Sedimentation Velocity (AUC-SV)
AUC-SV experiments are the gold standard in the characterization of HOS of proteins, large peptides, antibodies, nano-particles and polymers. Specifically, AUC-SV can be used to fully characterize the tertiary structure of before, during and after formulation. The results of these experiments will give the size distribution of proteins, polymers, nano-particles and antibodies; estimated molecular weights; calculated shape and the quantification of all the species in the system including HOS. Any differences in the sedimentation coefficients and the calculated shapes will provide insight into possible conformational changes both at the secondary and tertiary structure levels. The major advantage of AUC-SV over conventional size exclusion chromatography is that there is no dilution of the samples which can prevent self-association and HOS. Additionally, fast equilibrium self/hetero associations can be evaluated under the time scale of the AUC-SV experiment. Fast equilibrium self/hetero associations are usually not observed on the time scale of a SEC experiment which can lead to erroneous results and in accurate HOS characterization.
-
Size Exclusion Chromatography Multi-Angle Light Scattering (SEC-MALS)
SEC-MAL experiments will give the absolute molecular weights in solution and a size distribution profile. In addition to the obtaining absolute molecular weights, HOS, if present, can also be characterized.
-
Top Down Mass Spectrometry
Top down analysis to ascertain the molecular weights (intact mass analysis) of the biological sample can conducted using MALDI and ESI ionization techniques. Both ESI and MALDI are complementary techniques, however MALDI is a “softer” ionization technique which can provide information that might be lost during the ESI process.
-
Analytical Ultracentrifugation Sedimentation Velocity (AUC-SV)
-
Evaluation of Binding Affinities
Techniques available:
-
Isothermal Titration Calorimetry (ITC)
When mutations or chemical perturbations in a protein are present, one of the most important parameters that needs to evaluated, especially in the therapeutic environment, is its binding affinity. Changes in binding affinities are important makers in determining whether protein is acting in its wild type capacity. ITC is the gold standard when it comes for a direct measurement of binding affinities. ITC directly measures the association constant (Ka), thermodynamic parameter (enthalpy, dH) and the stoichiometry of the interaction.
-
Isothermal Titration Calorimetry (ITC)
Triclinic Labs Large Molecule Characterization Techniques and Data Derived
| Technique | Aggregation | Stability | PTMs | Primary Sequence | Interactions | Comments |
| Analytical Ultracentrifugation (AUC) | Quantitation of HOS*, Molecular Weights | Yes | No | No | Stoichiometry, stability of interaction and Kd | ICHQ6B |
| SEC-MALS | Quantitation of HOS, Molecular Weights | No | No | No | Hydrodynamically stable interactions only. | ICHQ6B |
| FT-Raman/IR | Assessment of HOS | Yes (Secondary Structure Perturbations) | No | No | Yes | ICHQ6B |
| HPLC | Quantitation of HOS | Yes | No | No | No | ICHQ6B, |
| FDA Biosimilarity** | ||||||
| LC-MS/MS-MS | No | Yes | Yes | Yes | No | ICHQ6B, |
| FDA Biosimilarity | ||||||
| ITC | No | No | No | No | Yes (Stoichiometry, Kd) | ICHQ6B |
*HOS, Higher Order Structures
**FDA Biosimilarity, Scientific Considerations in Demonstrating Biosimilarity to a Reference Product
Mass Spectrometry
(GC/MS, LC/MS, LC/MS/MS and MALDI) -
Structure elucidation
| Application AND Technique Description | ||
|---|---|---|
|
Used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for
elucidating the chemical structures of molecules, such as peptides and other chemical compounds. When skilfully analyzed,
MS spectra can provide important information for structural elucidation/characterization and facilitate identification of
unknown compounds by comparison to mass spectral libraries.
An important enhancement to the mass resolving and mass determining capabilities of mass spectrometry is using it in tandem with chromatographic and other separation techniques. A common combination is gas chromatography-mass spectrometry (GC/MS or GC-MS). In this technique, a gas chromatograph is used to separate different compounds. Similar to gas chromatography MS (GC-MS), liquid chromatography-mass spectrometry (LC/MS or LC-MS) separates compounds chromatographically before they are introduced to the ion source and mass spectrometer. It differs from GC-MS in that the mobile phase is liquid, usually a mixture of water and organic solvents, instead of gas. Mass spectrometry has both qualitative and quantitative uses. These include identifying unknown compounds, determining the isotopic composition of elements in a molecule, and determining the structure of a compound by observing its fragmentation. Other uses include quantifying the amount of a compound in a sample or studying the fundamentals of gas phase ion chemistry (the chemistry of ions and neutrals in a vacuum). MS is now commonly used to study physical, chemical, or biological properties of a great variety of compounds(e.g. large and small organic molecules and inorganics). |
||
| Instrument Brand | Model | Notes: |
|
Voyager
Agilent Agilent Finnigan Thermoquest |
DE Pro (MALDI)
5975C 6320 Trap LC/MS XL95 LCQ |
Wiki Reference for MS and Hyphenated Techniques
|
Biosimilar
In addition to routine characterization of large molecules and peptides, development of Biosimilar (generic versions of large molecule therapeutics) has increased significantly due to the patent expiration of major biologics (Graph 1). Legislation passed by Congress in 2009 and regulatory guidance provided by the FDA in 2015, has opened the door for potential market approval in the U.S. using an “abbreviated” regulatory pathway for biosimilars that does not follow the full requirements of section 351(a).
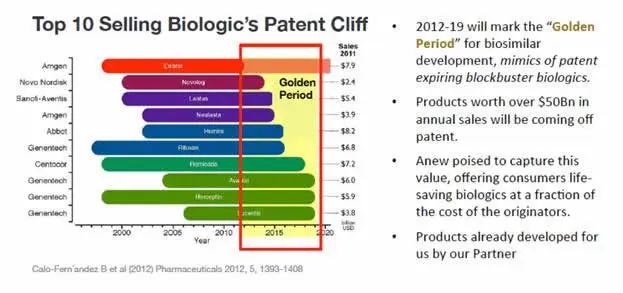
Graph 1. The effect of Biological Patent Expiration. Approximately 20% of the ~4,500 biopharmaceutical candidate products in the current pipeline (>900) are follow-on biopharmaceuticals — mostly biosimilars (>650), but also biobetters.8 As such, the FDA has developed a number of guidance documents in this area including Scientific Considerations for Demonstrating Biosimilarity to a Reference Product and Test Procedures and Acceptance Criteria for Biological Products. 9,10
A biosimilar characterization program should incorporate a strong analytical package that typically includes testing of the following properties:
- Protein quantity and purity
- Amino-acid sequence
- Post-translational modifications assessment
- Physicochemical properties, and
- Identification and quantitation of Higher Order Structures (HOS)
- Biocomparibility (ICH Q5E guidelines) during the manufacturing process, lot release and stability testing should also be incorporated.
- Analytical Method Development, Validation, Release, Transfer.
Protein quantity and purity.
A “pure” protein is one that is free from any quantifiable amounts of impurities. Variables such as the structural properties of the protein itself, the amount of protein available, the nature of potential contaminants in the sample, and the accuracy of the estimate required should always be considered when selecting the method of analysis.Purity is typically determined by HPLC, Size Exclusion Chromatography (SEC), LC-MS/MS-MS and should indicate the presence and/or absence of contaminants. Purity of a protein can also be measured at a UV absorbance of 280nm and by running a SDS PAGE gel followed by further staining to check for any impurities.
Analysis of the primary structure of the molecule.
Characterization of the amino-acid sequence (normally using LC/MS or LC/MS/MS-based approaches: peptide-centric bottom-up methods) is important to verify that the product produced is that as expected from the engineered gene sequence.Change in the primary structure of a protein could affect the down-stream higher-order composition, which could affect the properties and activity.
Determination of the primary amino sequence is of paramount importance as outlined in the ICH Q6B guidelines and for any type of Biosimilar characterization.
Post Translational Modification Analysis.
Post-transcriptional modifications (e.g., oxidation, acetylation, phosphorylation, glycosylation, lipid attachment and/or intentional modifications, such as PEGylation), should be thoroughly characterized using LC/MS/MS-based approaches as these can affect all forms of higher-order structure and can impact efficacy as well as immunogenicity.Aggregation, Stability.
Stability of the protein under development should be assessed at both the secondary and tertiary structural levels. Buffer composition, pH, additives such as sucrose, Tween 20/80 can all affect the stability of the protein. Changes in secondary structure at the dosage concentration can be evaluated using FT-Raman/IR and Circular Dichroism (CD). Additionally, lyophilization effects on the biological products can be easily evaluated using FT-Raman/IR (solid state analysis).Aggregation analysis (HOS) as either part of stability or bio comparability study during the manufacturing process can be assessed using Analytical Ultracentrifugation (AUC), Size Exclusion Chromatographic coupled to Multi-Angle Light Scattering (SEC-MALS) and HPLC methods. Both qualitative and quantitative assessment of the HOS of the protein can be conducted. A full characterization of the protein (analysis of self-association, dissociation constant (Kd) and rate of aggregation) should also performed if any higher order structures (HOS) are detected.
Assessment of Protein-Protein and Protein-Ligand Interactions
Characterization of reversible self and hetero-associations (protein-protein/ligand) using AUC to determine stoichiometry, stability of the complex and strength of the interaction (Kd) is necessary to understand the dynamic nature of the protein/s during the formulation process. Isothermal Titration Calorimetry (ITC) can be used orthogonally with AUC to evaluate the Kd and stoichiometry of system if a reversible binding partner is present.Analytical method development, transfer, and validation
Triclinic Labs is an industry leader in GMP method development, transfer, and validation. We offer services for the analyses of biological products and biosimilars and method development. Methods by HPLC, LC-MS, NMR, FTIR, and other techniques can be developed and validated. All methods are developed and validated according to ICH guidelines for accuracy, precision, specificity, limit of detection, limit of quantitation, linearity, range, and robustness. Methods can be transferred.For more information on these techniques please contact:
Aeri Park, Ph.D.
Vice President
apark@tricliniclabs.com
Phone: 765.588.6200
Direct: 765.588.9538
References:
-
Specifications:
TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS.
ICH Harmonised Tripartite Guideline. Q6B, Current Step 4 version, March 1999. -
Quality of Biotechnological Products:
STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS.
ICH Harmonised Tripartite Guideline. Q5C, July 1996. -
Stability Testing of New Drug Substances and Products.
ICH Q1A(R2), revision 2, November 2003. -
COMPARABILITY OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS SUBJECT TO CHANGES IN THEIR MANUFACTURING PROCESS.
ICH Harmonised Tripartite Guideline. Q5E, June 2005. -
SPECIFICATIONS:
TEST PROCEDURES AND ACCEPTANCE CRITERIA FOR BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS.
ICH Harmonised Tripartite Guideline. Q6B, Current Step 4 version, March 1999. -
Guidance for Industry:
ANALYTICAL PROCEDURES AND METHODS VALIDATION FOR DRUGS AND BIOLOGICS.
Pharmaceutical Quality/CMC. July 2015. -
Guidance for Industry:
BIOANALYTICAL METHOD VALIDATION.
Biotherapeutics. Revision 1, September 2013. -
Guidance for Industry:
QUALITY CONSIDERATIONS IN DEMONSTRATING BIOSIMILARITY OF A THERAPEUTIC PROTEIN PRODUCT TO A REFERENCE PRODUCT.
Biosimilarity. April 2015. -
13th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production.
BioPlan Associates, Inc.: Rockville, MD, April 2016;
www.bioplanassociates.com. ISBN 978-1-934106-28-0
