Start with exceptionally well characterized active pharmaceutical ingredients and excipients
Surface Science Services
(say that three times fast)
From particle size analysis to flow characterization, our analytical laboratory provides you with complete analysis and
reports tailored to your needs. Triclinic Labs offers complete Powder Characterization Services on certified and
calibrated equipment to assist you in characterizing your materials. We utilize surface science to assess surface,
physical and chemical properties as well as develop strategies for formulation with emphasis on dry powder functionality
either for oral or inhaler dosage forms. We have experience with tablets, hard and soft gelatin capsules, solid dispersions,
and self-emulsifying systems. We specialize in analysis of physico-chemical properties of powders and interactions among
powders (agglomeration and segregation) as well as surface properties as they relate to functionality of drugs and excipients.
Ask Our Scientists!
Request More Info
Our characterization services allow you to:
- Investigate particle-particle interactions and interactions of water vapor with different surfaces
- Understanding agglomeration and flow of particles
- Understanding adhesion of particles to processing equipment, substrates
- Quantify the effects of surface variability on product’s functionality in order to establish meaningful criterion for acceptance
- Access stability during formulation processing and storage
Understand Processes and Particle Phenomena induced by
- Milling
- Fracture & Disorder
- Drying
- Hot Melt Extrusion
- Spray-drying
- Powder Flow
- Segregation and Agglomeration
- Stability and Performance
- Solubilization and Dispersability
Full powder characterization including:
- Aerated and packed bulk density
- Air jet sieve
- Angle of repose, angle of spatula, cohesion, uniformity
- Bulk Density
- Contact Angle Measurement
- Light scattering analysis (0.04-200µm)
- Microscopy (optical, SEM)
- Particle size and domain analysis using Laser Diffraction Analysis (0.7-400µm)
- Rototap
- Sieve analysis
- Surface energy analysis
- Tap density
- Water Uptake (water isotherms) and Moisture analysis
- X-Ray sedimentation analysis (0.01 microns - 50 microns)
Ask us about:
- Blending and milling analysis using novel technologies (IGC, AFM, SEM, Confocal microscopy, DMA, vapor sorption, XPS and spectroscopy).
- Inline Sensor (NIR and Raman) usage in PAT approaches for assessing blend homogeneity and sameness of milled materials.
- Using quality-by-design for controlling materials, formulation, and processes to comply with the FDA PAT initiative.
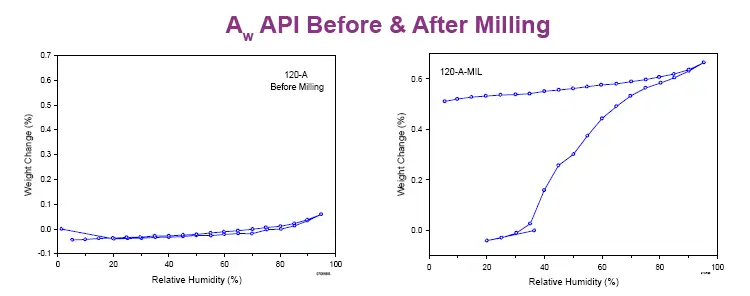
Figure 1. A batch of an active pharmaceutical ingredient before and after milling that passed all required physicochemical tests according to specifications but did not flow like other batches in the mill (i.e. it completely clogged the mill). We can utilize particle characterization to determine how early we can anticipate deviations from desired powder behavior upon milling, and avoid them. From the “The impact of water adsorption on the energetics of surface interactions of various powders.” in Water Properties of Food, Pharmaceutical and Biological Materials,, pp. 639-645 (2006). Carvajal
Service Levels Available:

