Analytical Method Development, Validation, and cGMP Release Testing -
Highly Sensitive Qualitative or Quantitative Analytical Method Development & Validation
Solid Mixture Specialists
Detection of low dosage API (< 1%) in complex formulations
We offer analytical chemistry method development and validation services for a wide range of solid-state analytical technologies, including the application of these approaches for pharmaceutical intermediates, APIs, excipients, contaminants, formulations, and finished drug product.


GMP Testing
We offer both cGMP (using a verified monograph test) and non-GMP testing We also offer custom method
development, method validation, GMP release testing, and method transfer services.
Triclinic Labs is the listed release lab in numerous agency filings. We are routinely audited by clients, are registered with a variety of regulatory agencies (FDA, DEA, Health Canada) and are highly experienced in solid mixture analysis.
-
Analytical Method Development
-
Method Development
- Dissolution Methods
- Spectroscopic Assays (Raman, FTIR, NMR)
- X-ray Powder Diffraction Analysis (Quant, Limit, ID)
- Chromatographic Assays (HPLC, GC)
- Particle Size Assays (Wet and Dry)
- Optical Rotation
- KF
- Thermal
- Method Validation
- Technology Transfer
- Detection limit testing
- Linearity testing
- Precision testing
- Repeatability testing
- Accuracy testing
-
Quantitation limit testing
-
Method Development
-
Contaminant Analysis, Patient Complaint Samples
- Foreign material identification
- Extractables/leachables identification
-
Root cause analysis
-
Compendial Testing
- USP/EP/JP/BP/PhEur Monograph Testing
- Raw material (API/Excipient) identification
- CoA issuance
- Limit Test for Impurities
- Assays for Drug Substances and Formulations
- System Suitability Testing
-
cGMP Batch Release Testing
- CoA issuance
- Reference standard generation and qualification
- Analyze finished products and bulk lots for purity, concentration, consistency, and identity.
Contact us for pricing on GMP Testing (monograph), Custom method development & validation, and cGMP release testing (click here)
Considerations for solid state methods:
Numerous types of methods are available for pharmaceutical analysis. These methods include qualitative analysis
such as discriminant analysis methods used for raw material identification as well as various types of
quantitative methods. Quantitative methods include limit test assays, predictive assays, and the underutilized
sunset assays. Published guidelines for analytical method development (ICH Q2) were written for liquid mixtures
and do not anticipate problems associated with solid mixtures. Factors affecting analytical responses from
solids, like particle size and crystal lattice flexibility, must be understood and accounted for. Also, use of
multivariate data and chemometric processing techniques is critical to establishing adequate method
sensitivities.
Triclinic Labs' scientists have developed many qualitative and quantitative solid-state analytical methods based on various analytical techniques such as x-ray powder diffraction, Raman and infrared spectroscopy, moisture sorption, solid-state NMR, and other measurements. These methods have included the determination of crystalline material in amorphous matrices, crystalline in crystalline assays, and amorphous in crystalline matrices. In particular, Dr. Bugay has a broad experience base in quantitative analysis and has lectured on the topic frequently. By using multivariate/chemometric data processing techniques, limits of detection and quantitation for solid mixture analytical methods can be significantly reduced compared to standard univariate (single-peak) methods.
Developing Methods
Triclinic Labs is able to support all stages of pharmaceutical development from lead optimization to manufacturing. Analytical methods can be developed and validated in accordance with either client-specific protocols or internal SOPs, and all developed methods are validated to meet FDA, MHRA, and/or ICH guidelines for:
- Linearity and Range
- Accuracy
- Specificity and selectivity
- Analyte Precision
- Intermediate Precision
- LOD / LOQ
- Ruggedness
- Standard and Sample Solution Stability
-
Robustness (optional)
Validation
The same analytical method development guidelines (ICH Q2) also provide guidance for validation of the various
assays, but unfortunately approach the discussions from a solution phase perspective, not for solid-state
mixtures. Triclinic Labs' scientists have used their extensive experience to validate the same solid-state
qualitative and quantitative assays that they have developed. Their experience in solid-state chemistry, the
respective analytical techniques, and validation requirements for solid-state assays can be depended upon for
truly accurate, precise, and robust methods.
A very good article on Method Development Approaches as outlined in the ICH Guidelines: Assay Development and Method Validation Essentials A 10-step systematic approach to analytical method development and validation can improve the quality of drug development. Nov 01, 2012 By Thomas A. Little, PhD BioPharm International Volume 25, Issue 11 .
The Science and Art of Quantitative Method Development and Validation for Drug Products
Developing a validated quantitative method for drug product can be a considerable challenge with a number of significant pitfalls if the method development is not scientifically sound. In addition to the scientific challenges are the challenges imposed by GMP and the requirements imposed by following the recommended ICH guidelines for quantitative method development. Within this section, discussion will be limited to the scientific considerations and in particular the scientific considerations required to develop a method for a solid state product. The ICH guidelines are more suited to liquid based assays and quantitative methods for liquids and do not address the unique problems associated with the solid state.Quantitative methods developed for the solid state have to allow for the influence of the solid state matrix on the analytical signal from each individual phase being quantified. If the matrix effect is the same for the calibration standards, validation standards and the drug product being analyzed, then the influence of the matrix can be largely ignored if the change in phase concentration is small. This is rarely the case in reality. While the calibration and validation standards may be mixed from the same source materials using the same methods for mixing and sample preparation, the drug product will almost certainly be manufactured using a very different process and often with different source materials. The expression 'different source materials' refers to different vendors of the same excipient/API or even different lots from the same vendor.
The primary physical properties that drive the matrix effect are particle size/shape, particle density and packing density - in short, anything that will influence the spatial homogeneity of the analytical signal produced by the sample material. A sample that exhibits a completely homogeneous distribution of analytical signal from all volume elements of the sample will have no matrix effect. For a solid state mixture to achieve this ideal, the particle size would have to be unrealistically small and the packing density unrealistically high; essentially forming a liquid. Although some drug product tends towards this ideal, the artificial standards made in a laboratory are far from ideal. Ironically, making standards of known weights in a non production line laboratory as outlined in the ICH guidelines, introduces the matrix error into a quantitative method intended for a drug product. To avoid the matrix error for a validated ICH quantitative method intended for drug product would require that all the calibration and validation standards be manufactured at scale using the same process and materials as used for the drug product. This is almost always an unrealistic expectation.
A quick estimate of the influence of the matrix effect on any particular method or drug product can be easily determined using the raw analytical signal from the initial calibration 'standards'. The matrix influence tends to split the individual phases that make up the drug product into one of two groups: 'heavy' phases or 'light' phases. Heavy phases show enhanced analytical signal levels and light phases show a reduction in the analytical signal level relative to the expected level for the known weight of the phase in the sample. The definition of heavy or light for phase is a relative term and will depend on all the phases in the drug product. Plotting raw analytical signal level (normalized to the mass of the standard being analyzed) against known weight for each of the phases should reveal a slight bowing of the plots away from linear. Heavy phases will show an upward deflection and light phases a downward deflection from the expected linear response. The magnitude of the deflection will give you the magnitude of the matrix error with respect to weight percent of each phase. This is not a random error but is systematic in its effect, shifting some phases to higher concentrations and others to lower concentrations.
Some drug product matrices have shown weight percent errors of 30% or more. The original calibration lines developed for the quantitative method may not show any departure from non-linearity if the range of concentrations is small or if the data has been pre-normalized by the quantitative method such as by the normalization procedures routinely employed by chemometric methods.
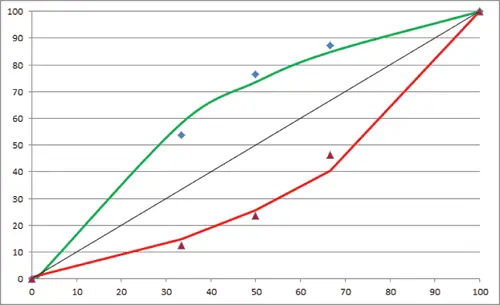
Figure 1. Calibration for a binary system consisting of an API and excipient of different densities. The bowing of the calibration line is as expected for a series of artificial standards using materials of different density, particle size and packing efficiency. Errors of up to 25% in absolute concentration are possible for binary materials close to 50:50 w/w.
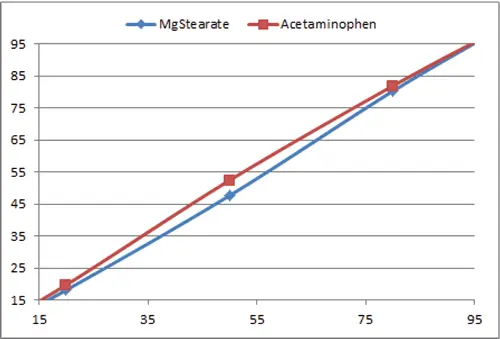
Figure 2. Bowed calibration line for a binary system of acetaminophen and magnesium stearate. The particle sizes were similar and the artificial standards were compacted to improve packing efficiency. In this case, the errors for a 50:50 w/w sample have been reduced to about 2%. These curves represent about the best that can be achieved for binary mixtures through careful preparation of the artificial standards.
Chemometric Software developed at Triclinic Labs is able to reconstruct the matrix influence using the bowing
of the calibration lines.
This enables the development of a quantitative method that follows the ICH guidelines but fully corrects for any
matrix errors built into the calibration through the use of artificial standards.
As a first approximation, the matrix error free calibration line can be employed directly to quantify drug product material assuming that the drug product is closer to the ideal matrix free sample than were the artificial standards. For many drug products, the drug product material introduces its own matrix error which can be corrected for with a second matrix model. The benefit of the matrix model as used at Triclinic Labs, is that it allows the development of a validated method following the ICH guidelines and then allows that method to be transferred for drug product quantification. If the production procedure is changed or different source materials are used to manufacture the drug product, a matrix model for the new drug product can be used to bring the quantitative model back into compliance.
Throughout this discussion of quantitative methods, the actual analytical techniques used have not been identified. It is our experience that the common solid state analytical tools used for quantitative analysis all show some degree of matrix effect. For some methods and materials, the effect is small but on average the matrix influence will give a significant shift to quantitative results whether the analytical technique used is X-ray powder diffraction or Raman or any of the common solid state quantitative methods.

Download our Whitepaper "Triclinic Labs Chemometric Engine" by Dr. Ting Xu for a full description of the software we use and its advantages in method development. Click Here.