We offer cGMP and non-GMP Particle Size Analysis, Particle Distribution, Counting, Property Assessment, and
Identification and Contamination services as well as GMP Method Development and release testing.
Particle size and particle distribution significantly impacts drug product efficacy as well as other organic,
inorganic, and metallic materials. Triclinic Labs has significant experience in both wet and dry particle size
and distribution analysis, method development, contaminant identification, property determination and support
of the following monographs:.
- USP <429> Laser Diffraction Measurement of Particle Size
- USP <616> Bulk Density (Method I) and Tapped Density (Method II) of Powders
- USP <695> Crystallinity
- USP <846> Specific Surface Area
- USP <776> Optical Microscopy (Particle Size, Particle Shape Analysis, Static Image Analysis and Crystallinity Characterization)
- USP <1174> Powder Flow
- USP <1241> Water-Solid Interactions in Pharmaceutical Systems - Determination of Sorption Desorption Isotherms
See below for more detail about the services and techniques we offer.
Please contact us about cGMP Particle Size Method Development, Validation, and Release testing.
Particle Size Analysis and
Particle Size Distribution -
Determine size and distribution of particles using Laser-Diffraction
| Application AND Technique Description | ||
|---|---|---|
In a laser diffraction particle size measurement, a laser beam passes through a dispersed particulate
sample and the angular variation in intensity of the scattered light is measured. Large particles
scatter light at small angles relative to the laser beam and small particles scatter light at large
angles. The angular scattering intensity data is then analyzed to calculate the size of the particles
that created the scattering pattern using the Mie theory of light scattering. The particle size is
reported as a volume equivalent sphere diameter. The particle-size distribution (PSD) of a powder, or
granular material, or particles dispersed in fluid, is a list of values or a mathematical function
that defines the relative amount, typically by mass, of particles present according to size (see
figure below) 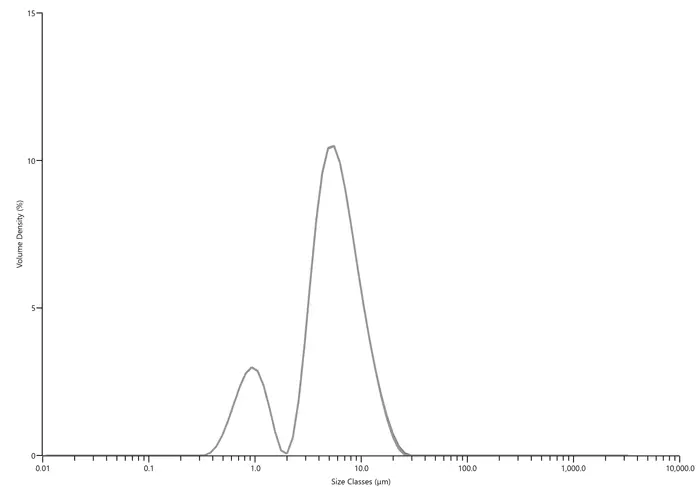
|
||
| Instrument Used | Model | Notes |
| Malvern |
Mastersizer 3000 v.3.70
Malvern Access Configurator v.2.20 Particle Analysis Ranges: Dry Range: 0.1 - 3500um Wet Range: 0.01 - 1400um |
cGMP and non-GMP testing available.
We offer cGMP method development, validation, and release testing as well as method verification and transfer capabilities. Contact us for details. 
cGMP Analysis and Method Development Available |
RAMAN Spectroscopy -
Identify molecules, determining particle morphology and composition of a component in a mixture, study chemical bonding, spatially locate chemicals
| Application AND Technique Description | ||
|---|---|---|
|
Raman is a spectroscopic technique used to observe vibrational, rotational, and other low-frequency
modes in a system. Raman spectroscopy is commonly used in chemistry to provide a structural
fingerprint by which molecules can be identified. A sample is illuminated with a laser beam.
Electromagnetic radiation from the illuminated spot is collected with a lens and sent through a
monochromator. Elastic scattered radiation at the wavelength corresponding to the laser line (Rayleigh
scattering) is filtered out by either a notch filter, edge pass filter, or a band pass filter, while
the rest of the collected light is dispersed onto a detector.
We specialize in hyper-spectral imaging or chemical imaging, in which thousands of Raman spectra are acquired from all over the field of view. The data can then be used to generate images showing the location and amount of different components. Raman spectroscopy is suitable for the microscopic examination of minerals, polymers and ceramics, forensic trace evidence, organic and inorganic materials, and to differentiate polymorphic forms of active pharmaceuticals. With Raman microscopy you can:
|
||
| Instruments Used | Model | Notes: |
|
Renishaw
Thermo Ondax |
Dispersive Raman
: inVia Chemical imaging, microsampling, Confocal, 785 nm laser. Morphologically Directed Raman
Spectroscopy (MDRS)
FT Raman : Thermo FT Micro and macro sampling, 1064 nm laser, Omnic v.9.7.46 software with spectral libraries. Low frequency : Ondax THz-Raman® With Probe Stokes and anti-Stokes signals from ±5 cm-1 to 200 cm-1, (or 150 GHz to 6 THz) |
cGMP and non-GMP testing available.
Wiki Reference for Raman spectroscopy

cGMP Analysis Available |
Hall Flow Rate Testing -
Powder flow rate and density
| Application AND Technique Description | ||
|---|---|---|
|
This technique determines both flow rate and apparent density. It is applicable to both free-flowing
metallic powders and other fine powders testing. The testing is performed with a calibrated hall
flowmeter funnel according to International Standards.
Used in: Powder metallurgy, metallic powders, food powders, cement powders, plastic powders, rubber powders, ceramic powders, pharmaceutical powders, foundry, casting, metalworking. |
||
| Instrument Brand | Model | References |
| Hall FlowMeter | AS-300 | |
Particle Charge Analysis -
Identify handling issues due to charge
| Application AND Technique Description | ||
|---|---|---|
|
Powders and other materials can acquire an electrical charge on the surface of their particles due to
contact and movement against processing and handling equipment and containers. Contact, friction, and
movement of particles within the material itself can also cause charge acquisition.
It is important to measure particle charge since this can lead to problems and unstable flow and powder behaviors. Charged materials stick to processing equipment and storage systems, can become airborne more easily, and can flow in different ways than materials with no charge. |
||
| Instrument Used | Model | References |
Density Testing -
Density is the mass of a substance with regard to the space in which it occupies. It is important to determine the density when there are changes in vendor materials or in processing.We offer the following density testing: (True, Apparent, Bulk, Tapped, Carr index)
Tapped and Bulk Density -
Explore powder flowability and settlement
| Application AND Technique Description | ||
|---|---|---|
|
The bulk density of a material is the ratio of the mass to the volume (including the inter-particulate
void volume) of an untapped powder sample. The tapped density is an increased bulk density attained
after mechanically tapping a container containing the powder sample. The tapped density is obtained by
mechanically tapping a graduated measuring cylinder or vessel containing the powder sample. This
technique is particularly useful in powder flowability studies and also in determining the amount of
settlement. The compressibility index and Hausner ratio are measures of the products ability to settle, and permit an assessment of the relative importance of inter-particulate interactions. In a free-flowing powder these interactions are less significant and the bulk and tapped densities will be closer in value. For poorly flowing materials, there are greater inter-particulate interactions and a greater difference between the bulk and tapped densities will be observed. The differences are reflected in the compressibility index and Hausner ratio. |
||
| Instrument Used | Model | References |
| Caleva | TDT 12 | WHO Reference for Bulk and Tapped Density |
Other Testing Services Available:
- Charge
- Porosity
- Sieve Analysis
-
Release Testing
Contact us for further details or pricing.
