Improve Compound Solubility using Non-Crystalline (X-ray Amorphous) Systems and Amorphous Solid Dispersions (ASD):
Some low solubility compounds will dissolve in gastric fluids if the dissolution rate can be increased, therefore micronization (particle size reduction) may lead to success in those cases. Other compounds will never dissolve no matter how small the particle size is. An increasing percentage of new chemical entities (NCEs) exhibit poor aqueous solubility which results in poor bioavailability and those properties cannot be improved with traditional solid-state techniques (salt selection, cocrystal development, micronization). These molecules may be suited for development of an amorphous dispersion by spray drying, hot melt extrusion, or other methods.Triclinic offers services to guide amorphous material development, determine stability of materials produced in a variety of ways (e.g. varying formulations), and can develop validated assays to determine if the material is amorphous or contains crystalline components.
Polymorph screening is commonly conducted on an API for the purpose of understanding its polymorphic landscape. Naturally, within a screen there is a motivation to identify metastable and stable polymorphs at a given temperature. Long-term stress or slurries are commonly done to evaluate stable polymorphs. On the other hand, a variety of techniques can be used to screen for metastable polymorphs. One popular approach for screening for metastable forms is to prepare the material in a higher energy state through generation of amorphous material and use that as a starting material in crystallization experiments. Various techniques can be used to prepare amorphous material including melt-quench, rotary evaporation, and/or lyophilization. Melt-quench experiments do not utilize solvent and avoid any complications around solubility or degradation in solution; however, the decomposition temperature of the API must be reasonably higher than its melt for the melting experiment to work. In rotary evaporation experiments, the evaporation occurs from a bulk solution, which can be detrimental to the preparation of amorphous material as it allows API molecules to re-orient and crystallize. Lyophilization experiments typically require longer times to prepare and is only suitable with a limited number of solvent systems with adequate solubility of the API and a freezing point above the lyo operating temperature.
On the other hand, spray drying is a technique which rapidly evaporates the solvent from solution micro-droplets, thus there is much lower opportunity to crystallize compared to the evaporation techniques from a bulk solution (albeit crystallization rate is highly dependent on the API molecule itself). At Triclinic, we have a ProCepT spray dryer which can be utilized to prepare amorphous material on a small scale, i.e., <500 mg.
Approximately 60% yield has been shown for several experiments to date and depending on the volume of the solution each spray drying takes <5 minutes (although setup, startup, and cleanup take about an hour total). Thus, due to the ease of the technique, amorphization via spray drying should be evaluated as part of polymorph screening, if possible.
Good candidate molecules for spray drying should have the following:
-
Melting T >180 °C (this would give us Tg above RT based on the
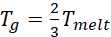
calculated in K)
- Solubility of at least 10-50 mg/mL in spray drying solvents (MeOH, EtOH, IPA, acetone, EtOAc, MEK, water) à other solvents like DCM, chloroform, THF are not preferred since they are not compatible with the tubing, although mixtures with the preferred solvents could be attempted
The spray dryer can achieve up to 40-50 °C temperature drop between the initial (drying gas temperature) and
collection stage. Please consider this in relation to solvent’s boiling point and the API’s Tg.
At Triclinic, the following considerations are evaluated in ASD development:
- API: Is the API a fast or slow crystallizer? What is the solubility advantage of the amorphous form?
- ASD: Which polymer from a toolbox of 8-12 proven additives, is best? What is the best drug loading (%w/w)?
- Processing: Should the ASD be generated via solvent- or melt-based approach?
- Stability: What are the recommended storage conditions? How does temperature and humidity affect stability?
- Performance: Does the ASD show superior performance during in vitro dissolution compared to its crystalline counterpart?
Triclinic Labs is equipped with a ProCept R&D spray dryer and a MiniCTW hot melt extruder. The entire ASD development can be performed with as little as 500 mg material. In addition, Triclinic Labs is equipped with complete thermal, microscopy, and spectroscopy techniques sensitive for crystallization detection needed in an ASD development. Contact us today for more information (click here).
Why Triclinic?
Triclinic has more than two decades of experience in finding new solid forms, evaluating their physico-chemical properties , and selecting the best form for commercialization. We've collaborated with companies large and small on thousands of polymorph, salt, amorphous material, and cocrystal projects.- The scientists at Triclinic have performed more the 300 cocrystal screens to date
- Our hit rate is extremely high (e.g. 75% of the 100 APIs we screened in the last 12 months resulted in at least one cocrystal)
- We have the only staff, laboratory, screening process, and equipment that has been dedicated exclusively to solid form screening for the past 15 years
- We have industry leading proven proprietary screening techniques (e.g. well-plates) that are only available at Triclinic. We constantly update and improve our screening approaches.
- Our database of pharmaceutically acceptable guests and coformers is more diverse and better researched than any other
What's it cost and how long does it take?
Ahhh - the magic (and usually final) questions! Without knowing the molecule's properties, what has been done to date, and the goals of the project, it is difficult to estimate total project cost and timelines. But we know you need those numbers. So we usually have a short discussion with you and build a customized proposal to meet your time and project goals. There's no cost involved and we can usually generate a quote within a few days. So please contact us:
Utility of Low Frequency (LF) Raman Mapping: Dissolution of Acetaminophen (ACE) Spray Dried Dispersions (SDD).
Nico Setiawan, Ph.D., Andrew Smith, and David E. Bugay, Ph.D.The latest approaches for developing and characterizing non-crystalline (amorphous) materials.
This application note outlines the evaluation of post-dissolution ACE SDD. The results indicated that in all of the cases studied, ACE remained amorphous upon dissolution if it was intimately mixed in the dispersion. However, phase separation from the polymer allowed ACE to crystallize as expected. Interestingly, the type of polymer used affected the ACE polymorph generated. The mixture of polymorphs was observed in the powder X-ray diffraction (PXRD) result (though it could easily be overlooked), while LF Raman maps showed distinct regions of the different polymorphs. This study demonstrated the utility of LF Raman mapping in elucidating the dissolution mechanism of amorphous dispersions.
Keywords: Low Frequency Raman, Amorphous, Spray dried dispersion, Dissolution, Crystallization, Polymorphs
Download The Complete Technote here:
Novel Non-Crystalline Materials Analysis: New Strategies to De-risk Amorphous Material Formulation Development.
Simon Bates, Ph.D.The latest approaches for characterizing non-crystalline (amorphous) materials and determining physical stability under typical storage conditions.
Amorphous forms consist of disordered arrangements of molecules that do not possess a distinguishable crystal lattice. Different forms of a drug substance can have different chemical and physical properties, including melting point, chemical reactivity, apparent solubility, dissolution rate, optical and mechanical properties, vapor pressure, and density. These properties can have a direct effect on the ability to process and/or manufacture the drug substance and the drug product, as well as on drug product stability, dissolution, and bioavailability. Instability of non-crystalline materials is of particular concern to regulatory bodies within companies and national agencies.Download The Complete Technote here:
