Ensure Your Regulatory Submissions are Complete...
Test your ingredients for identity, strength, quality, and purity:
Compendial (pharmacopeial) testing on the materials used in the manufacturing of pharmaceuticals is a basic requirement for most regulatory submissions around the world. Established pharmacopeial monographs such as those from the US Pharmacopeia-National Formulary (USP/NF), the European Pharmacopoeia (EP), the Japanese Pharmacopoeia (JP), and the British Pharmacopoeia (BP) provide standardized methods and specifications for pharmaceutical raw materials and finished products. Triclinic Labs has extensive experience with API, excipient, intermediate, and finished product analysis. We utilize USP/NF, EP, JP, BP, AOAC and ACS compendial methods for raw material and final product testing. Triclinic Labs provides a certificate of analysis (C of A) and operates under cGMP guidelines. We are FDA inspected (2016) and registered, and DEA licensed (Schedules I-V).


Our cGMP and non-cGMP material testing services include:
Complete Compendial Limit and Physical Testing (USP, EP, BP, JP, FCC, ACS, Others)
-
General Identification Tests (USP <191>)
- TLC Identification Test (USP <201>)
- Residual Solvent Testing (USP <467>)
- Melting Range or Temperature (USP <741>)
- Loss on Drying (USP <731>)
- Dissolution (USP <711>)
-
Identification and quantification by chromatographic procedures (TLC, GC, HPLC, GC/MS, HPLC/MS/MS)
- HPLC, GC and IC Analyses (USP <621>)
- Spectrophotometric Analyses / Infrared absorption (FTIR, UV/VIS) (USP <197> F, K, M)
- Metals Analyses (AA, GFAA, ICP) Container Testing (USP <661> and <671>)
- Uniformity of Dosage (USP<905>)
- Karl Fisher Moisture Analyses (USP <921>)
- pH (USP <791>)
- Other Wet Chemical Analyses (e.g. Color, Bulk Density)
Applications for Compendial Testing:
- Raw material testing (USP/NF, EP, JP, BP, Other International Pharmacopeial Organizational Standards)
- Dissolution studies
- ICH Stability Studies (raw material and finished products)
Applications for Non-Compendial Testing:
- Method Validation and/or transfers
- Verification of Raw Material received
- Impurity and Contaminant identification (for additional info click here)
- Stability and Disintegration studies
- Problem Solving (e.g. variable lot dissolution, consistency)
Method Development, Validation, and Consultation
It is common for our customers to require alternative methods to established compendial procedures. We have not only the capacity to perform these alternative methods, but also provide method development and validation services. For validation guidelines we use ICH (International Conference on Harmonization), EP (European Pharmacopoeia) and USP (United States Pharmacopeia). These guidelines specify the typical analytical characteristics required for validation testing. Depending on the particular test method and purpose (assay, impurity, degradation product, limit test, etc.), validations may include accuracy, precision, specificity, detection limit, linearity, range and robustness.
We are available to help you decide what the appropriate approaches are to meet your Compendial and Non-Compendial testing objectives.

Are you ready for FDA / USP <901> Talc Regulatory Compliance Testing?
Talc is mined from the ground, and normally, there are veins of asbestos near the talc, offering the possibility of some potential contamination.
On December 26, 2024, the FDA proposed a rule to standardize how asbestos is detected in
talc-containing cosmetics. In parallel, the United States Pharmacopeia (USP) has overhauled its
Talc <901> monograph - effective December 1, 2025 - to strengthen asbestos detection in
pharmaceutical-grade talc by formally coupling X-ray powder diffraction (XRPD) with polarized light
microscopy (PLM) methods. Beginning this year, to certify talc as USP grade, each batch must be
screened under these new requirements.
At Triclinic Labs, we are committed to ensuring the safety and compliance of pharmaceutical talc products by
offering comprehensive asbestos detection services. In alignment with the United States Pharmacopeia's (USP)
General Chapter <901> Detection of Asbestos in Pharmaceutical Talc, our laboratory utilizes
state-of-the-art analytical techniques to identify and quantify asbestos contaminants accurately.
To keep you ahead of the curve, we have verified the new USP method and offer GMP-validated methods
for
testing talc for the presence of asbestos in pharmaceutical and other products:
-
XRPD: This technique assesses the overall purity of talc products by identifying
accessory minerals and determining the presence or absence of total amphibole and serpentine minerals.
We have redundant powder diffractometers that offer rapid turnaround time for cGMP analysis of your
starting material.
- PLM: Complementing XRD, PLM detects asbestos particles at lower levels and differentiates between various asbestos types, providing a detailed analysis of potential contaminants.
Triclinic Labs ensures that your pharmaceutical talc products meet safety and regulatory requirements by
adhering to USP's rigorous standards and employing advanced methodologies. Our experienced team delivers
precise and reliable results, supporting the integrity of your products and safeguarding public health.
Contact us
today for sample pricing, turnaround times, and other details about Talc testing (click
here).
For more information on USP's General Chapter <901>, please visit the official USP notice:
https://www.uspnf.com/notices/gc-901-prospectus-20210430
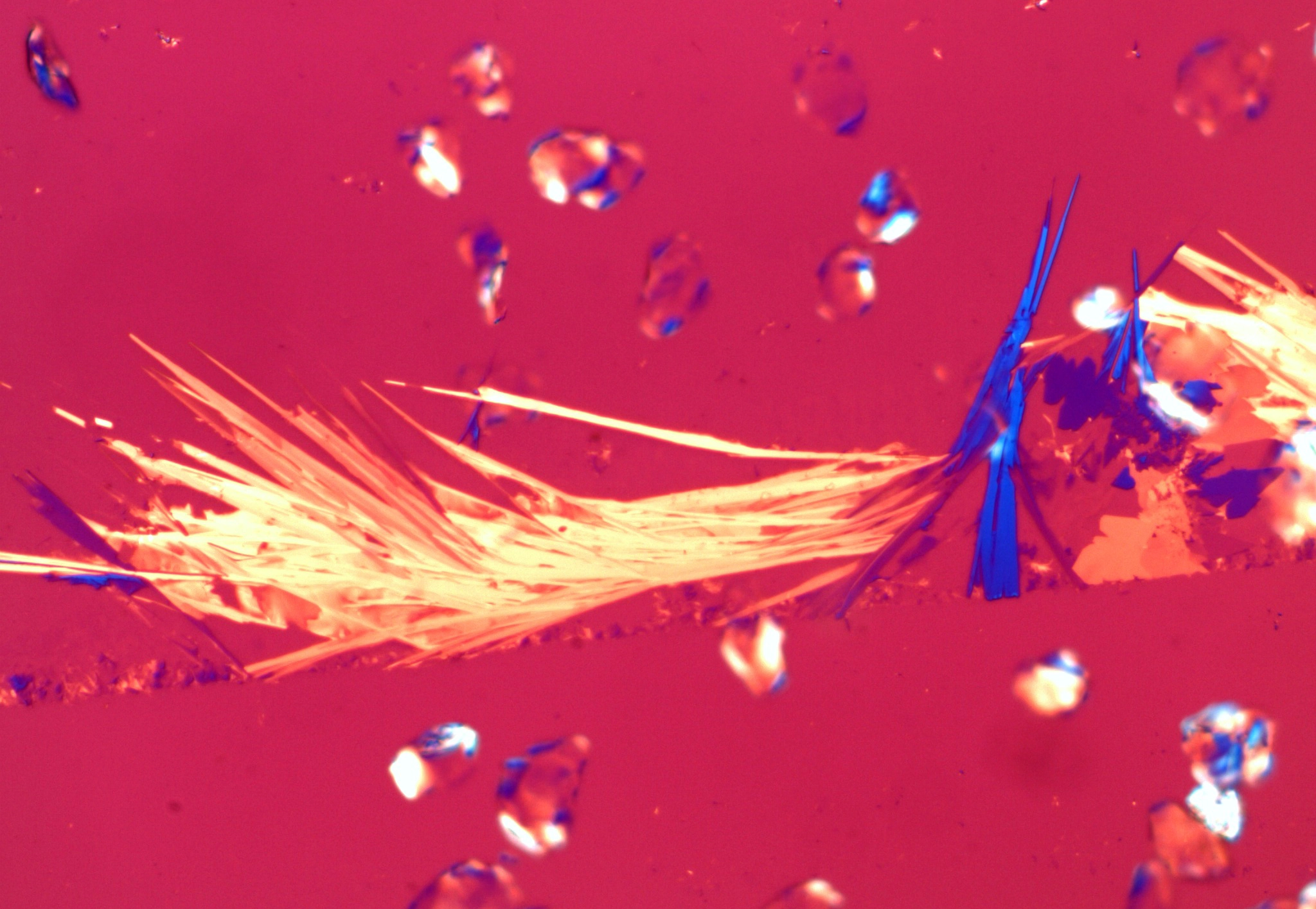 Suspected asbestos fiber found in "USP grade Talc". Shown in 1.550 RI liquid taken with a 10X
objective at a rotation angle of 90° Suspected asbestos fiber found in "USP grade Talc". Shown in 1.550 RI liquid taken with a 10X
objective at a rotation angle of 90° |
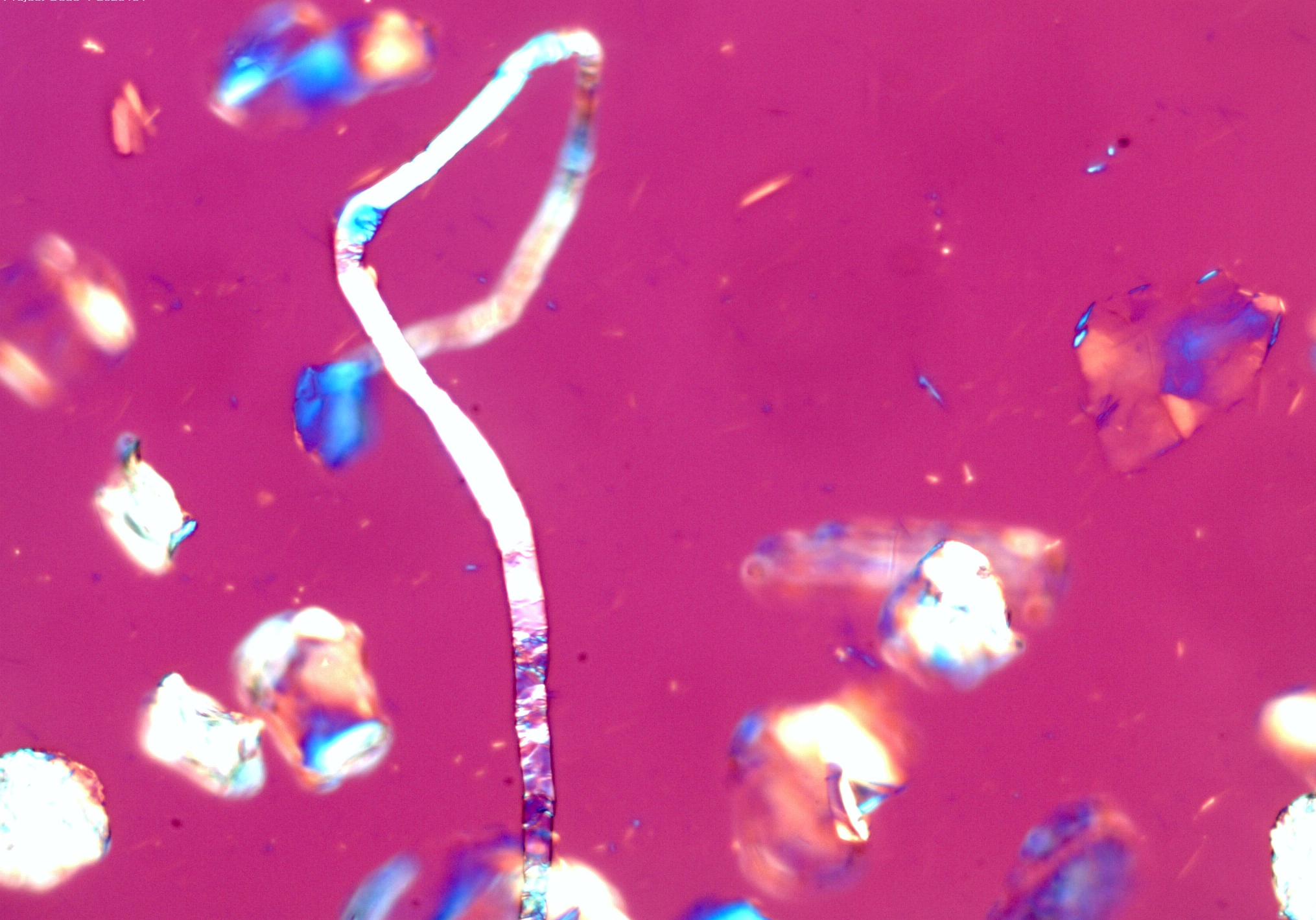 Suspected asbestos fiber found in "USP grade Talc". Shown in 1.605 RI liquid taken with a
20X objective at a rotation angle of 180° Suspected asbestos fiber found in "USP grade Talc". Shown in 1.605 RI liquid taken with a
20X objective at a rotation angle of 180° |
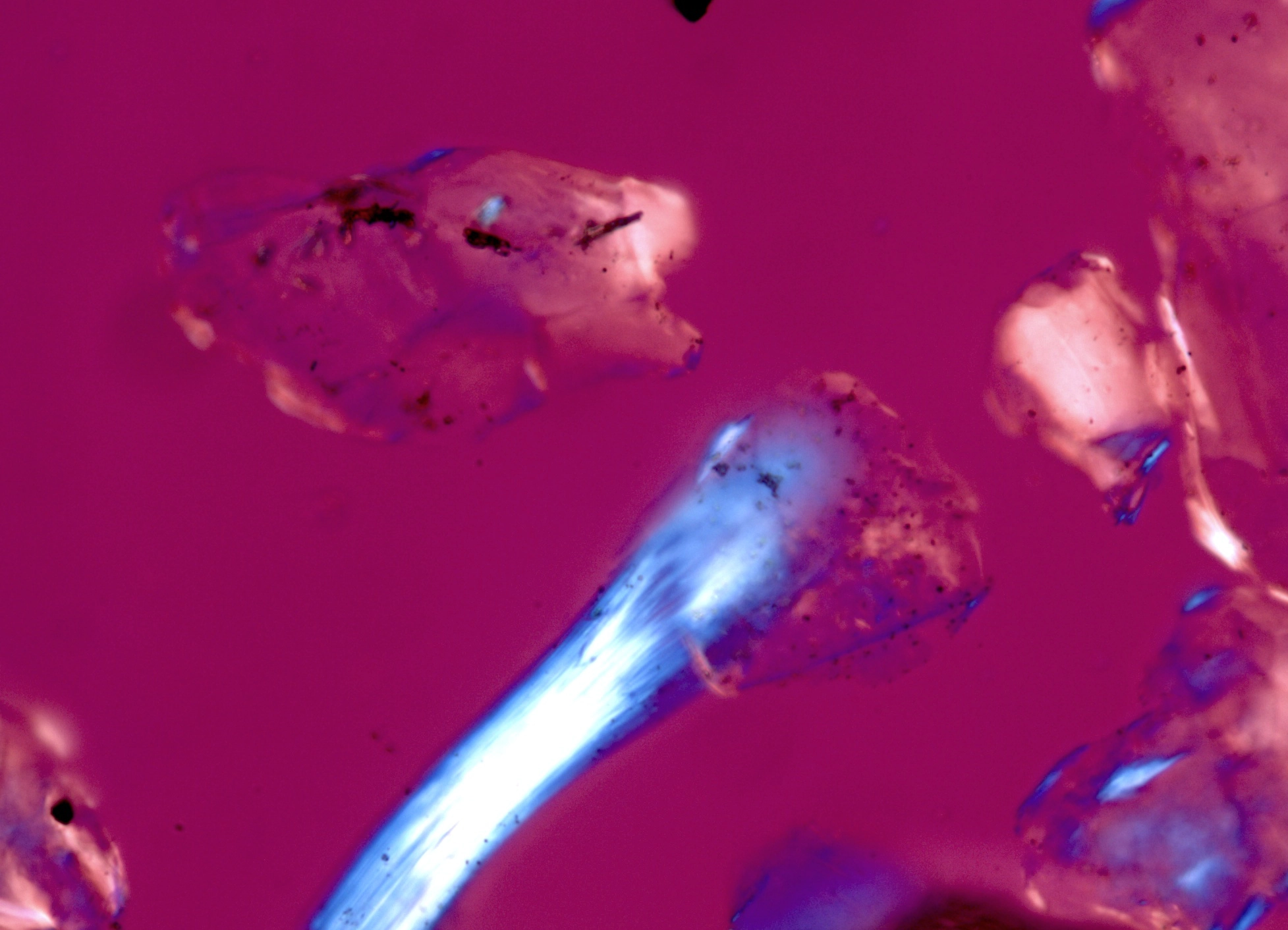
Suspected asbestos fiber found in a consumer talc product. Shown in1.550 RI liquid taken with a 40X objective at a rotation angle of 180° |
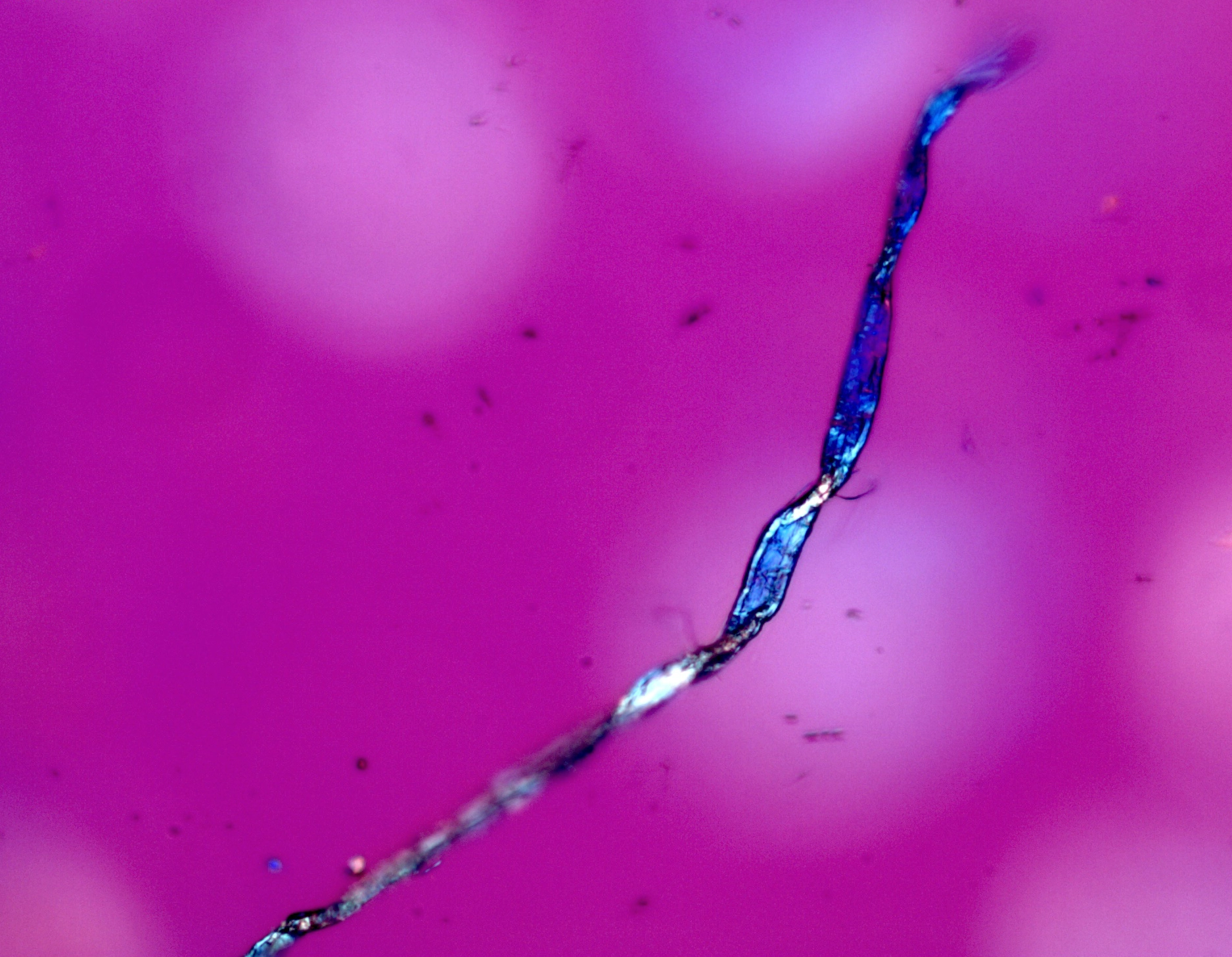
Suspected asbestos fiber found in a consumer talc product. Shown in 1.605 RI liquid taken with a 20X objective at a rotation angle of 180°. |
Figure 1. Polarized light microscopy images of commercially available talc samples - The top two images are from a USP grade bottle of talc. The bottom two images are from a consumer talc product.
