Find crystalline polymorphs and salts that improve chemical properties, solve problems, ensure consistent material for development, and expand intellectual property...
Different crystalline solid forms (polymorphs, salts, cocrystals) of an active pharmaceutical ingredient (API) can have very different physico chemical properties. Those differences can impact bioavailability, solubility, dissolution rate, first pass metabolism, side effect incidence, stability, API and drug product manufacturability, and other important parameters. The vast majority of APIs can exist in multiple solid forms, including polymorphs, hydrates, solvates, salts, cocrystals, and non-crystalline forms. The goal in screening and selection is to produce an optimal solid form based on parameters such as crystallinity, stability, solubility, hygroscopicity, and ease of production ("manufacturability"). Understanding the crystallization process is paramount for reproducibility during drug development and manufacturing...Triclinic has extensive experience in solid-state and solid form screening & selection, crystallization methodology, and in devising and conducting studies to improve your drug's physico-chemical properties. Some reasons an alternative solid form may be desirable:
- If you are searching for a developable form of your API we can determine pharmaceutical polymorphism, the most stable form, as well as develop pharmaceutical salts, cocrystals, and non-crystalline materials.
- If your API has poor aqueous solubility , there are types of solid forms that can be found and patented that will lead to improved solubility.
- If your drug has poor stability or handling properties , Triclinic can recommend approaches to improve those properties.
- If you are a generic company or innovator looking for an improved version of a generic drug to patent and market for increased profitability and franchise extension, Triclinic can suggest strategies to produce the improved product through use of novel, patentable solid forms.
- If you need solid form life cycle management approaches, searching for and patenting new solid forms several years before patent expiration of a drug is a valuable technique. Triclinic can carry out studies that will result in novel forms and lead to development of improved, patentable product extensions.
While there is no guarantee that your chemical will be polymorphic or be able to generate a usable salt, but data collected by Dr. Pat Stahly at Triclinic Labs indicates the prevalence of alternate solids forms is likely:
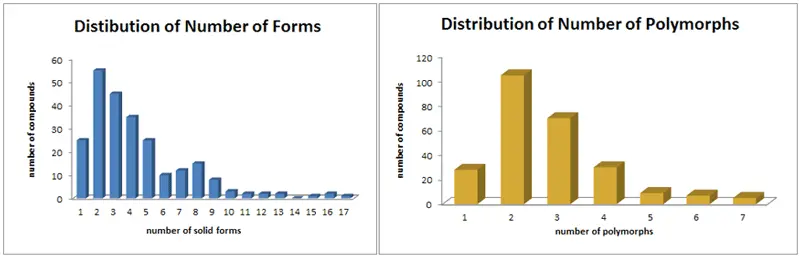
Contact us for a free consultation on improving your asset's solid form and intellectual property.
We offer the following services for Solid Form Development:
Polymorph Screening and Selection
Polymorphism is the existence of a chemical in two or more crystalline phases that have different three-dimensional arrangements and/or conformations in the crystal lattices. In general, a polymorph screening study is a search for those distinct crystalline phases. The physical and chemical properties of each solid form (polymorph) of an API can vary dramatically and have a significant impact on the pharmacokinetics, consistency and ease of manufacturing, and overall product stability. In many cases, one of the goals of a polymorph study may be a robust crystallization process that consistently produces the desired polymorphic form of the API during all phases of manufacturing.Properties that can differ among solid forms (polymorphs) of molecules include:
- Color
- Compressability
- Crystal shape (habit)
- Density
- Filterability
- Flow
- Hardness
- Hygroscopicity
- Particle Shape
- Particle Size
- Solubility
- Stability
Energy differences between forms are usually relatively small and as such, form interconversion is common and can occur during routine API manufacturing operations, drug product formulation, storage and use. Stability of the molecule is of particular concern because manufacturing conditions often produce an unintentional and incorrect form due to introduction of thermal and mechanical energy. Scale up of a process often leads to encountering new forms and inadvertently in the later stages of development. This can delay clinical trials, manufacturing campaigns, and regulatory submissions.
Polymorphs have intellectual property advantages and a comprehensive screening study should be done to ensure franchise protection and a complete portfolio. There is also significant consideration in claims construction with respect to polymorphism and solid form characterization.
Polymorph screens are often conducted at various stages of development.
Salt Screening and Selection
If polymorphs of the chemical asset do not exist, the form does not have the desired properties for development (e.g. solubility, stability, crystallinity), and the molecule is ionizable, a pharmaceutical salt screen can be conducted to ameliorate these issues. Pharmaceutically acceptable counterions are selected for screening along with knowledge of the molecule. Screening projects are designed in a tiered manner and with a variety of techniques to improve on the properties of the API that are problematic.Counterion selection is based on:
- pKa of the API
- Previous clinical and market use
- Toxicology data
- Past use and success in screening studies
-
ICH Guidelines
Cocrystal Screening and Selection
Like salt screening, cocrystal screening is usually undertaken when another suitable solid form cannot be found and/or the molecule is non ionizable. Cocrystals take advantage of guest molecules in a crystal lattice along with the API and often the solubility of the guest enhances the cocrystal's overall solubility. Guests are selected from a wide variety of sources such as pharmaceutically acceptable and Generally Recognized As Safe (GRAS) lists. Triclinic has more than 200 guest molecules available for screening. Cocrystals are often used for purification and stabilization as well as intellectual property improvement. Existing pharmaceuticals with physical or chemical property issues can be improved as well.For a complete discussion on cocrystal screening, selection, and formulation please click here for a more extensive review.
Amorphous (Non Crystalline) Material Development and Solid Dispersion Development
Amorphous materials are generally much more soluble than crystalline materials, and can be formulated to be physically and chemically stable throughout the shelf life of the drug product. Triclinic has pioneered materials modeling approaches for amorphous materials and can provide insight in how to improve the manufacturing process, formulation process, and predicted shelf life of solid dispersions manufactured in a wide variety of ways.For a complete discussion on non-crystalline (amorphous) materials please click here for a more extensive review.
Crystallization Method Development and Troubleshooting
A crystalline API needs to exhibit consistent physical properties when made at each stage of the development process (gram to multi-kilogram scales). In doing so, formulation-development problems are minimized and regulatory requirements are met. Triclinic is experienced in generation of crystallization methods, including the background information necessary to impart solid form control (polymorphic form, particle size, etc). crystallization in chemistry, Chiral Resolution, and chemical Purity. When problems arise in commercialization or scale up, we can quickly help you get your program back on track by identifying a robust and scalable method to consistently control the desired solid form during manufacturing.For a complete discussion on crystallization method development and its applications, please click here for a more extensive review.
Chiral Material Characterization and Resolution
Solid forms developed from a chiral compound and purified from racemic mixtures can be crystallographically unique. Solid-state racemic mixtures may exist as a racemic compound (a racemate) where enantiomers are present within a single crystal structure, or as a conglomerate (a physical mixture of two crystal phases in equivalent amounts) each containing single enantiomers. We have extensive experience in both the characterization of these materials as well as development of approaches for resolution.Frequently Asked Questions About Solid Form Screening and Selection:
What's an overall strategy for solid form screening and selection?
We believe that regardless of the name you apply to the material, the properties are what are most important to development success. Here's our approach to the overall screening and selection landscape:
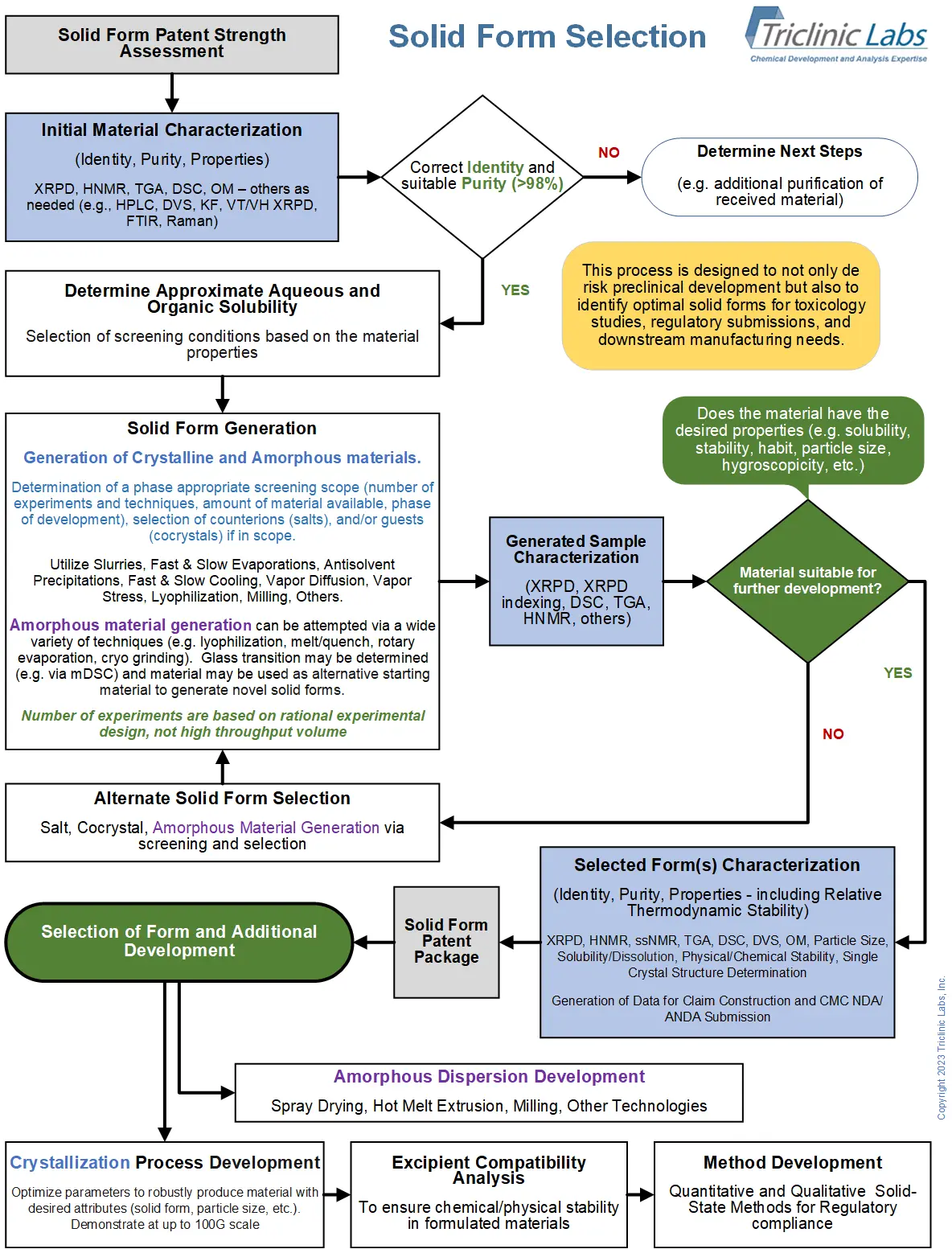
When in the drug development cycle should you conduct pharmaceutical polymorph screens and how hard should you search?
In the pharmaceutical industry, regulations require that polymorph screening be carried out during API development to determine if the API can exist in polymorphic forms and, if so, whether the choice of polymorph will affect the drug's efficacy or safety.
We suggest solid form development should begin prior to toxicity studies so that you can determine the magnitude of differences in toxicity as it relates to different levels of solubility of the solid forms you have. Therefore, when you test the first human exposures, you have a general idea if the form that you're administering is going to be representative of your final drug product.
Beyond that, a search for solid forms of an API is often conducted to overcome production, formulation, or drug product problems caused by the form produced initially. Triclinic Labs' scientists have performed thousands of polymorph screens and problem solving exercises to control solid form during API manufacture, formulation, storage, and use. We can develop a screen suited to your compound's individual requirements and stage of development. Whether you seek initial indication of polymorphism or complete screening for intellectual property use, we can customize experiments and levels of characterization to meet your goals. Typically we conduct the following screens:
- Preliminary Polymorph Screen (Search for most stable form, includes screening, competitive slurry experiments with forms, characterization of new forms found)
- Extensive Polymorph Screen (Comprehensive, screening for IP coverage using the widest variety of search conditions)
- Minimal API Polymorph Screening (Done in micro plates when sample availability is <1gram of material)
An incomplete understanding of your active ingredient’s solid-state properties may lead to unintended and unnoticed solid form conversion during scale up, processing, or storage. These changes may affect manufacturability, bioavailability, solubility, stability, and clinical outcomes. The use of different polymorphic forms during clinical development is a common problem and may require bridging studies to demonstrate clinical trial validity to the regulatory authorities. Active ingredient solid-state properties should be investigated sooner rather than later. It is essential to mitigate risk by having a base understanding of the solid form landscape of your compound but conducting extensive screens too early can lead to unnecessary costs. The flow diagram below describes Triclinic Labs’ recommendation for when to conduct solid form screening and the reasons for doing so.
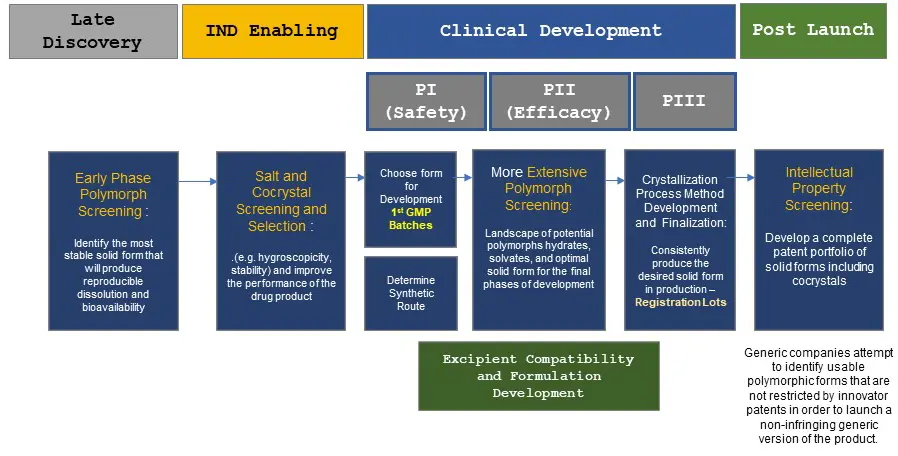
Do you advocate the use of high-throughput experiments? and How does the use of high-throughput technology impact the cost of performing crystallization experiments?
The number of usable samples that can be generated in a screen depends somewhat on the physical properties
- particularly solubility - of the test compound. For example, if the test compound exhibits poor
solubility in a wide range of solvents, it is likely not worthwhile to attempt generation of 150 samples,
as the techniques available for sample generation are limited. One could always slurry a compound in
various solvents in which it is insoluble to attain a target number of samples, but such experiments are
quite unlikely to yield new solid forms. Based on reports in the literature, isolable solid polymorphs
tend to be created in at least single-digit percentages of samples made. Thus, it is reasonable to target
less than 100 experiments, or maybe between 100-200 experiments, in a polymorph screen. The variety of
sample generation conditions is more important than numbers of experiments!
For salt and cocrystal screening, the number of experiments it makes sense to generate is related to the
number of counter-ion sources or coformers that are used. Typically two or three experiments with each
counter-ion source/coformer are all that are required.
While some companies employ High-Throughput Screening (HTS) technologies and claim it revolutionizes
screening by minimiizing hte starting material amoutn and reduces costly overhead, we disagree. Science is
unpredictable and a mcahine cannot adjust its experiements on the fly. A HTS setup simply perfomrs the
same experiemnts over and over. Often leading to unusable data or results. We've explored HTS technology
exensively over the last twethy plus years (including commercial systems) and have consistently found the
quality of results or the array of forms found do not
in any way compare to a well trained scientist with extensive screening experience.
What about Artificial Intelligence (AI) or Large Language Model (LLM) tools for predicting solid
forms?
Like all emerging tools, they can be helpful for guiding empirical approaches or developing strategies for
design of experiments. They are not a substitute for well planned laboratory experiments, however. We have
employed AI based approaches for a long time and while they can be useful, they suffer from an inability to
outpace empirical data sets and human problem solving. In our hands they have never produced a usable form
that has not been observed in the lab. They are quite good at producing comparative models and refinement
procedures for space groups and unit cell determination, but again - not a substitute for real human
intelligence and ingenuity. What current AI tools are unable to do is deploy real time laboratory
experimental adjustments that a well trained and experienced scientist can exploit. You cannot ask an AI
tool to craft a pot from clay. It can describe how to do it, but the technique and artistry is learned,
executed, and perfected by human hands.
What solvents and conditions are used in polymorph screening to identify and characterize new solid forms?
In polymorph screening, various solvents with distinct properties are employed to discover and characterize new solid forms. The process typically involves experimenting with a wide array of solvents, such as water, ethanol, methanol, acetone, and ethyl acetate. Each solvent offers unique interactions with the active pharmaceutical ingredient (API).
During the screening, these solvents are used under multiple conditions to explore potential outcomes:
-
Temperature Variations: Trials are conducted at different temperatures to observe how heat might influence crystallization or hydrate formation.
-
Pressure Adjustments: Some studies involve varying pressure settings to uncover new polymorphic forms.
-
Evaporation Techniques: Solvent evaporation rates are adjusted to see how they affect the solid state of the compound.
-
Concentration Levels: Different solvent concentrations are tested to identify the most effective mixture for crystallization.
These efforts aim to uncover distinct polymorphs, hydrates, or solvates and to understand their interrelationships, providing crucial insights into their potential applications and stability.
What if I need a pharmaceutical salt?
Salts of APIs are often used when the API itself is not sufficiently soluble or stable, or is difficult to
formulate or manufacture. In addition, different salt forms can have different bioavailability profiles or
organoleptic properties. When considering making salts of an API, one needs to select appropriate counter
ions based on a variety of factors such as, but not limited to, frequency of use in approved drugs,
dissociation constants, expected salt solubilities, and so forth. Triclinic scientists can plan and carry
out an effective salt selection project considering those factors, the particular properties of an API, and
its intended use. Salt selection for drug development at Triclinic employs an extensive list of counterions
and techniques.
Current thinking is that pharmaceutical salts represent one end of a structural continuum, with pharmaceutical cocrystals at the opposite end ( see Childs, S. L.; Stahly, G. P.; Park, A. Molecular Pharmaceutics 2007, 4, 323-338 click here for a discussion ).
What about a Cocrystal?
Cocrystals (or co-crystals), which are unique crystalline structures containing multiple components, have been known since 1844. The use of cocrystals containing pharmaceutical components was reported as early as 1895. Cocrystals have unique properties and have been shown to be stable and useful in pharmaceutical development. Advances have been made recently in methods of finding cocrystals and in generating them reproducibly using standard crystallization conditions. Pharmaceutical companies are developing cocrystalline forms of new APIs, and some companies are searching for patentable cocrystals of generic or soon-to-be-off-patent APIs in order to establish intellectual property positions. Triclinic is uniquely qualified to carry out cocrystal screening and aid in the patenting and development of those found.

For a complete discussion on cocrystal screening, selection, and formulation please click here for a more extensive review.
Can you develop Non-Crystalline Forms? (Amorphous Materials and Solid Dispersions)
Yes. Amorphous (Non-crystalline) forms of compounds dissolve more rapidly than crystalline forms, and can significantly increase bioavailability of poorly-water soluble APIs. However, the use of non-crystalline materials requires confidence that crystallization will not occur during product lifetimes. In addition, organic compounds do not necessarily exist simply in 'amorphous' and 'crystalline' states. Situations are known where X-ray amorphous forms of an API have different physical properties that depend on their method of generation. We can help you not only understand your non-crystalline materials and their properties but also provide confidence in stability over time. We also have services to take the amorphous material and develop a dispersion (formulation).

For a complete discussion on non-crystalline (amorphous) materials and their development please click here for a more extensive review.
Why should I choose Triclinic to conduct solid form screening and development instead of an API manufacturer or another company?
We're specialists, not generalists and we have extensive experience. Triclinic has more than four decades of expertise in finding new solid forms, evaluating their physico-chemical properties, and selecting the best form for commercialization. We can provide insight into problems encountered throughout development and help you build an intellectual property strategy around your chemical assets. Our company focus is solid-state chemistry for pharmaceutical and fine chemical development.
We've collaborated with companies large and small on thousands of polymorph, salt, and cocrystal projects.
We are thought leaders on state-of-the-art crystallization techniques and use
- Solvent-based (common and proprietary approaches)
- Slurry competition
- Thermal
- Sonication
- Grinding and solvent drop
- Well plate screening
- Phase diagram guided approaches
- Kofler technique and hot-stage
- Pressure
- Vapor deposition
- Novel nucleation techniques (e.g. use of polymers, inhibitors, and accelerators)
- Pattern matching and novel clustering algorithms
If you would like to discuss our services, please fill out the form below and one of our solid form screening experts will contact you.

DOWNLOAD OUR NEWEST SOLID-FORM SCREENING TECHNIQUES WHITEPAPER

Application of Low-Frequency (LF) Raman Spectroscopy to an Isoenergetic Polymorph Study
By G. Patrick Stahly, Ph.D.Typically x-ray powder diffraction (XRPD) is the most significant and first-choice method utilized to differentiate polymorphic forms of a substance, due to the fact that XRPD probes molecular packing directly, an XRPD pattern being a fingerprint of a specific three-dimensional arrangement of molecules. Other techniques probe crystalline structure indirectly, and therefore are occasionally unable to clearly differentiate polymorphs. For example, differential scanning calorimetry (DSC) discriminates by detection of different melting points, which are a consequence of different crystalline structures. Vibrational spectroscopic techniques, such as infrared (IR) and Raman spectroscopy, can sometimes be used to differentiate polymorphs based on the effects of molecular packing on molecular bond vibrational energies.
Download The Complete Whitepaper here:
