Experts in elemental and isotopic analysis of organic and inorganic materials
Elemental analysis is a process where a sample is analyzed for its elemental (and sometimes isotopic) composition. Elemental analysis can be qualitative (determining what elements are present), and it can be quantitative (determining how much of each element is present).
There are numerous techniques for analyzing elemental composition.
Please see below for an overview
We offer both cGMP and non GMP testing services. Please see below for more detail about the services we offer.


Elemental analysis -
Detection, identification, and quantification:
Elemental analysis and testing includes identification and quantification of elements, elemental compounds and molecular species. Sample types and matrices tested for trace elements include organic and non-organic, aqueous and non-aqueous materials. Elemental trace and ultra-trace analysis detection ranges from parts per million (ppm), to parts per billion (ppb) and parts per trillion (ppt) levels, using proven techniques.| Application AND Technique Description | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Elemental analysis is a qualitative and quantitative process for identifying the elemental composition
of materials (e.g., chemical compounds, minerals, metals, fluids). Certain elemental techniques can
even identify the isotopes of a given element.
We offer several elemental analysis techniques: Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a technique that can identify elements from sodium to uranium in solids, liquids, and aerosol filters. ICP-MS is a type of mass spectrometry that uses an Inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It is known and used for its ability to detect metals and several non-metals in liquid samples at very low concentrations. It can detect different isotopes of the same element, which makes it a versatile tool in Isotopic labeling. Compared to atomic absorption spectroscopy, ICP-MS has greater speed, precision, and sensitivity. In the pharmaceutical industry, ICP-MS is used for detecting inorganic impurities in pharmaceuticals and their ingredients. Chapter <232> Elemental Impurities - Limits; defines the maximum limits of fifteen elements in pharmaceutical products and Chapter <233> Elemental Impurities - Procedures; defines how the testing for these elements should be performed. These chapters have caused an increase in the need for ICP-MS testing. Previously, other analytic methods had been sufficient.
Energy Dispersive X-ray - (EDX) is a rapid technique for identifying the elements from beryllium to uranium in solid materials. EDX uses an electron beam to stimulate the emission of characteristic x-rays of the elements from the sample surface. The elemental results are presented in an x-ray spectrum. EDX can be used to analyze localized areas or generate chemical maps. Both qualitative and quantitative analyses can be performed. (Triclinic Labs typically uses EDX for the identification of contaminants.) EDX is very good for elemental analysis or chemical characterization of a sample with high levels of ionic elements (carbon, oxygen, copper, silver, aluminum, etc.) - including relative elemental composition information. Downsides include:
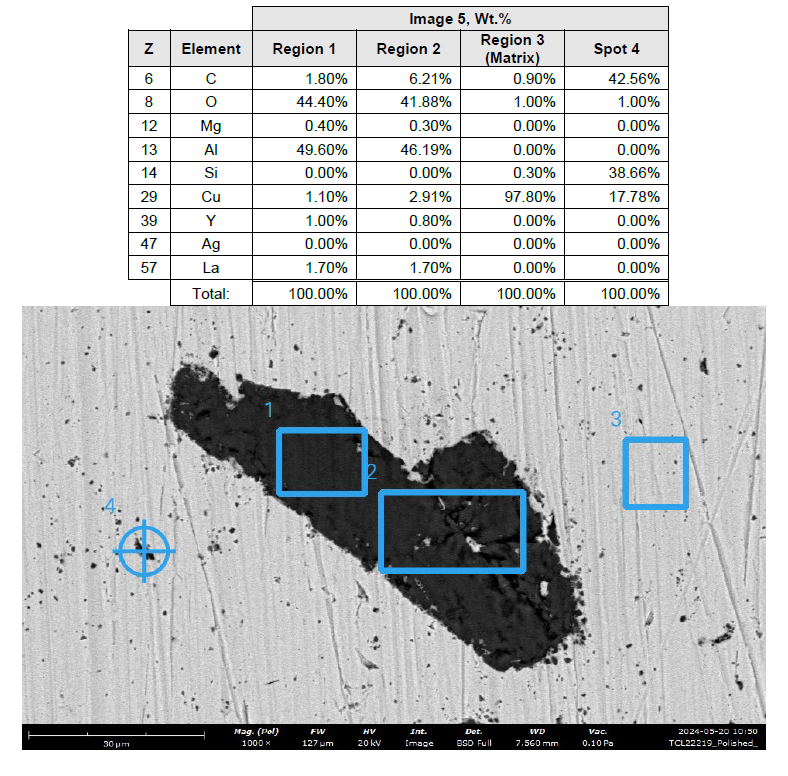
Figure 1. Foreign particle SEM image with spot (#4) and regional (#1,2,3) elemental analysis and resulting elemental composition shown in table. Region 3 demonstrates copper substrate and the inclusion body (regions 1, 2) contains aluminum, oxygen, copper, lanthanum, and carbon. A second smaller particle (4) contains carbon, silicon, and copper.
Figure 2. Synthetic sample showing EDX false color imaging. Magenta crystals are calcium carbonate, green crystals are calcium sulfate (gypsum), and the lilac crystal is graphite.
X-ray fluorescence (XRF)
is a
non-destructive analytical technique
used to determine the elemental composition of materials, primarily inorganics and simple organic
molecules. XRF analyzers determine the chemistry of a sample by measuring the fluorescent (or
secondary) X-ray emitted from a sample when it is excited by a primary X-ray source. Each element in
a sample produces a set of characteristic fluorescent X-rays (“a fingerprint”) unique
for that specific element, which is why XRF spectroscopy is an excellent technology for qualitative
and quantitative analysis of material composition.
 Proton Induced X-Ray Emission (PIXE) is a non-destructive technique that can identify elements from sodium to uranium in solids, liquids, and aerosol filters. It uses a proton beam to excite an electron shell change to force the emission of detectable X-rays. PIXE can be used to analyze multi-element samples because each element's X-rays are characteristic of the element and do not overlap with each other.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
| Instruments Used | Model | Notes: | |||||||||||||||||||||||||||||||||||||||||||||||||||
|
Thermo |
iCAP RQ ICP-MS |
This innovative single quadrupole (SQ) ICP-MS is the ideal trace elemental analyzer for a wide range
of sample types.
Method Development, Validation, Release Testing. |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermo | Phemom XL SEM/EDX | Fully integrated elemental and BSE detector | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Panalytical | Epsilon 4 ED-XRF | Spectrometer for the elemental analysis ranges from carbon (C) to americium (Am), and the concentration ranges from sub-ppm to 100 wt%. | |||||||||||||||||||||||||||||||||||||||||||||||||||