Can't find a salt? How about a cocrystal...
Cocrystals (or co-crystals), which are unique crystalline structures containing multiple components, have been known since 1844. The use of cocrystals containing pharmaceutical components was reported as early as 1895. Cocrystals have unique properties and have been shown to be stable and useful in pharmaceutical development. Advances have been made recently in methods of finding cocrystals and in generating them reproducibly using standard crystallization conditions.Pharmaceutical companies are developing cocrystalline forms of new APIs, and some companies are searching for patentable cocrystals of generic or soon-to-be-off-patent APIs in order to establish intellectual property positions. Cocrystals have been used to create crystalline forms of APIs that were previously known in only amorphous forms, improve melting points, enable improved chemical purity, reduce chemical degradation when exposed to light, remove the risk of hydrate formation in a formulation, and improve the solubility to enable higher bioavailability.
Cocrytals can offer significant physico-chemical property improvement!
Cocrystals have been developed in our lab to improve:
- Aqueous solubility
- Dissolution
- Hygroscopicity
- Highly Disordered Desolvated-Solvates (A volatile solvent can be replaced with a stable coformer in order to stabilize the crystallinity of the API)
- Highly lipophilic APIs
- Bioavailability
- Stability
- Increase of Melting Point
- Purity of API
- Intellectual Property Positions
- Developability
At Triclinic our focus on being the leader in the cocrystal field and our continuous innovation separates us from other CROs offering cocrystal screening services:
Triclinic now offers an expanded guest list for cocrystal screening and selection. The list of more than 250 coformers (all GRAS) is part of our STANDARD cocrystal screen and employs a variety of techniques for generating cocrsytals and solid samples. All hits can be further characterized and the results delivered in as few as 4 weeks including report.
We are the only contract research group to employ a phase diagram-guided approach to cocrystal screening. This, combined with our extensive coformer list results in a discovery rate of ~75% that leads the industry.
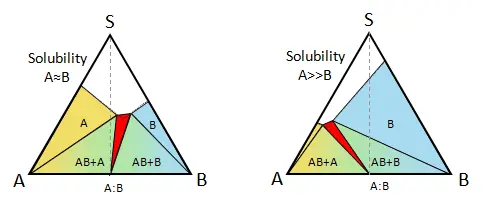 Figure 1.
Establishing phase relationships can lead to a more intelligent approach to solid-form screening and
development as well as prediction of undiscovered solid-forms or instability. Important tools in establishing
phase relationships are thermal analysis techniques such as differential scanning calorimetry (DSC),
thermogravimetric analysis (TGA), and increasingly, temperature-dependent XRPD. New phases are characterized
by their melting points and their stoichiometric domains.
Figure 1.
Establishing phase relationships can lead to a more intelligent approach to solid-form screening and
development as well as prediction of undiscovered solid-forms or instability. Important tools in establishing
phase relationships are thermal analysis techniques such as differential scanning calorimetry (DSC),
thermogravimetric analysis (TGA), and increasingly, temperature-dependent XRPD. New phases are characterized
by their melting points and their stoichiometric domains.
No other lab can match our experience and expertise in the field of cocrystals. If you want the industry leading cocrystal screen for your API, set up a consultation with Triclinic experts to discuss your project.
Our approach to Cocrystal Screening and Selection
Triclinic is uniquely qualified to carry out cocrystal screening and aid in the patenting and development of those found. We are globally recognized experts in the cocrystal field and carry out cutting-edge research to improve understanding of the structure and properties of cocrystals and to develop new cocrystallization methods.An important factor in the success of our screening process is the choice of reaction conditions.Reactions can be broadly classified as using stoichiometric or non-stoichiometric amounts of API and coformer.Stoichiometric reactions are performed as variations of a wet milling or solvent drop grinding process while non-stoichiometric reactions are a type of slurry conversion experiment.A wide variety of methods for facilitating nucleation and growth of a cocrystal are employed, including thermal, mechanical, and sonication processing.Solids are isolated and XRPD and/or Raman data from the solids are used to identify hits.Manual screening in individual vials is also performed for coformers that are not compatible with the parallel reactor technology. Triclinic scientists are also highly proficient at binary melt (Kofler) screening.This thermal technique is an effective manual screening method as well as a valuable characterization tool. Our standard cocrystal screen employs ~100 pharmaceutically-acceptable coformers but up to 250 such coformers can be used in a comprehensive screen. An extensive collection of coformer data, including toxicological and polymorphism information, enables us to design API-specific projects as well as rapidly asses analytical data to determine which screening reactions are successful.
Supersaturation Control and Formulation
An innovative aspect of Triclinic's approach to cocrystal formulation is identification and control over
supersaturation levels achieved during cocrystal dissolution. That can be achieved by directly
manipulating the cocrystal solubility using pharmaceutically-acceptable excipients that are incorporated into
a supersaturating drug delivery system. By using those excipients to tune a system for optimal
performance, the highest practical supersaturated solution concentrations can be achieved while avoiding
precipitation of the API.
The two phases of cocrystal formulation development are designed to 1) create and then 2) maintain API supersaturation.
These two phases are often referred to in the literature as the "spring" and "parachute".
1) Setting the Spring
Delivering APIs in ways that provide super-equilibrium concentrations constitutes the spring. A variety of formulation approaches have been used, including solubilized API, solid dispersions, nanoparticles, and inorganic carriers. The API can provide the spring itself if delivered in a high-energy form such as a non-crystalline solid or a cocrystal. Cocrystals offer the advantage over non-crystalline solids that the problem of physical instability to crystallization is avoided. To determine if a cocrystal provides a spring, well-designed, non-sink dissolution studies should be carried out. We have found that in general dissolution rates can be tuned based on the water solubility of the coformer, although the relationship of coformer water solubility to cocrystal water solubility is not always linear.
2) Opening the Parachute
The key to use of a supersaturating drug delivery system is to open the parachute, maintaining the concentration delivered by the spring long enough for absorption to occur. When using cocrystals, the spring concentration levels, the ratio of API and cocrystal solubility, the cocrystal dose, and the API crystallization rate must be considered. For example, the higher the supersaturation level delivered by the spring, the more driving force exists for API crystallization. Use of crystallization inhibitors in the formulation can delay API crystallization and increase the time over which supersaturation is maintained. (Figure 4). In another example, higher cocrystal loading in a formulation does not necessarily lead to higher exposure. Since lower loading provides a lower supersaturation level, API crystallization may be slowed (Figure 5).
Figure 3. Crystallization inhibitors are used to create a formulation that prolongs the supersaturated state. Inhibition of API crystallization in combination with a high supersaturation level can lead to higher plasma levels.
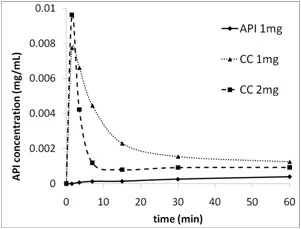
Figure 4. A formulation containing 1 mg of cocrystal AUC of 0.095 (0-30 min) whereas a formulation containing 2 mg of cocrystal had an AUC of only 0.051. The higher dose caused a correspondingly higher degree of supersaturation that led to more rapid crystallization rate of the API. The process of evaluating the dose effect in dissolution experiments is an important aspect of the formulation process.
Improved Development Results
Cocrystals have unique aspects that make them behave quite differently compared to polymorphs and salts, and
this makes the solid form selection process atypical for cocrystals. We have seen viable cocrystals rejected
from the development process because the routine characterization and analysis techniques used for evaluating
dissolution rate were incorrectly applied to cocrystal systems. Triclinic has the hands-on experience with a
wide variety of real-world cocrystal systems to help you avoid similar pitfalls. We have dedicated scientists
that work exclusively on cocrystals, and working with Triclinic will bring this experience and focus to your
team and improve the cocrystal development process at your company.
Purdue Pharma Example from McNamara et. al.Pharm. Res 2006, 23, 18
Dr. Scott Childs worked extensively with Dr. Dan McNamara of Purdue Pharma to improve an API whose bioavailability was unacceptable. A cocrystal screen was performed using the methods previously described and one candidate was taken forward for further evaluation. A better dissolution profile (Fig 6) and subsequent bioavailability improvement in dogs (Fig 7) was seen. In that case a fortuitously slow API crystallization rate allowed higher plasma levels to be achieved by administration of neat cocrystal.
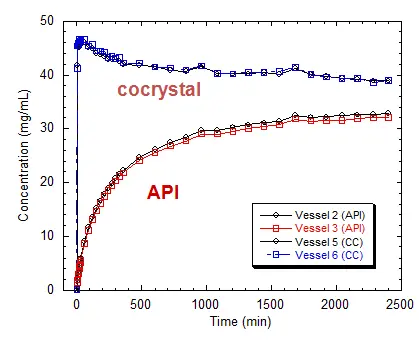 Figure 5. Intrinsic Dissolution (37 °C in water), The cocrystal
dissolves 18× faster than API
Figure 5. Intrinsic Dissolution (37 °C in water), The cocrystal
dissolves 18× faster than API 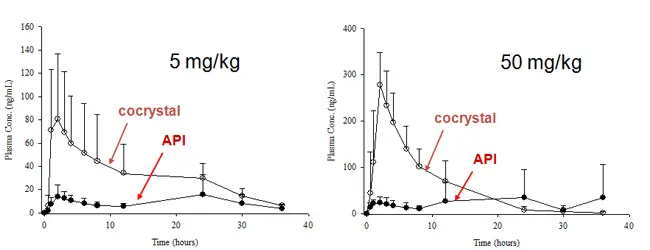
Figure 6. Bioavailability study in dogs demonstrates ~4X AUC increase.

- For a historical review of cocrystals see Dr. Stahly's article in Crystal Growth & Design 2007, 7, 1007-1026 here
- There is also an article by Dr. Childs et al. in JACS 2004, 126(41), 13335-42 on a Crystal Engineering Approach To Forming Cocrystals here.
What does the FDA say about cocrystals?
In April 2013 the FDA issued its final regulatory guidance on cocrystals, including the classification of a pharmaceutical cocrystal as a “drug product intermediate.”
Under this guidance, cocrystals are classified in a manner similar to polymorphs of a drug, rather than as salts. Under current guidelines, the polymorphs of an active pharmaceutical ingredient (API) are considered to be the “same” API, unlike unique salts that are treated as different APIs from a regulatory perspective.
There are intellectual property (IP) ramifications associated with the guidance as well. A cocrystal has the same active ingredient as the original API. Under the existing patent protection of a drug substance, a cocrystal version of the same molecule cannot circumvent the original patent. However, a cocrystal with new and more desirable properties can be patented, and ultimately marketed in the context of “lifecycle management”. Because cocrystals are considered to be the same drug substance under the new guidance, generic drug companies are able to introduce them as competitive products that are still considered to be the same API. In contrast, the use of a different salt would not be substitutable as the same active ingredient.
Drug product intermediates also have different regulatory reporting requirements compared to salts or polymorphs. Coformers used to make a cocrystal are defined by the guidance as excipients, which can form a simple physical mixture with the API or interact with the API on the molecular level (i.e. within the crystal lattice). The FDA guidance on cocrystals therefore requires assurance that complete dissociation of the API from its excipient (in this case a coformer) occurs prior to reaching the site of action for pharmacological activity.
Many in the industry find it surprising that the FDA has not classified cocrystals as new APIs as their properties can vary widely according to the conformer used. The current classification also makes it easier for generic companies to compete with branded products by utilizing cocrystals.
Why Triclinic?
Triclinic has more than four decades of experience in finding new solid forms, evaluating their physico-chemical properties , and selecting the best form for commercialization. We've collaborated with companies large and small on thousands polymorph, salt, and cocrystal projects in every therapeutic area.- The scientists at Triclinic have performed more than 500 cocrystal screens to date
- Our hit rate is extremely high (76% of the 200 APIs we screened in the last 24 months resulted in at least one cocrystal)
- We have the only staff, laboratory, screening process, and equipment that has been dedicated to cocrystal screening for the past 10 years
- We have industry leading proven proprietary screening techniques that are only available at Triclinic. We constantly update and improve our screening approaches.
- Our database of pharmaceutically acceptable coformers is more diverse and better researched than any other
- Our cocrystal publications have had an industry-wide impact and have the highest collective number of citations. To view our experts' Bibliographies on Cocrystals, please click here.
- We have in house experts and the most complete crystallography systems of any lab (X-ray, Electron, Powder, Synchrotron, Single Crystal, and Micro ED)
- We collaborate with the leading academic cocrystal research groups
If you would like to discuss our services, please fill out the form below and one of our solid form screening experts will contact you.


Cocrystals: A Regulatory Rebirth
By Pat Stahly, Ph.D.
What is the impact of recent changes in FDA guidance on regulatory and intellectual property issues?
The FDA reclassification of cocrystals as active pharmaceutical ingredients (APIs) has created exciting opportunities for the pharmaceutical industry. That reclassification means cocrystals can now be developed as if they were polymorphs, providing new avenues for dealing with poor API physical properties and extending intellectual property lifetimes. It is important to understand how the revised guideline impacts API development, regulatory submissions, and intellectual property protection. Those topics are discussed in this whitepaper. Also considered is the importance of identifying whether an API is a salt or a cocrystal, a critical classification from a regulatory point of view. In addition, a list of FDA-approved, marketed cocrystals is presented. Finally, recommendations for cocrystal screening, evaluation, formulation, and production at scale are given.
Download The Complete Technote
