Improve Compound Solubility using Non-Crystalline (X-ray Amorphous) Systems and Solid Dispersions:
Some low solubility compounds will dissolve in gastric fluids if the dissolution rate can be increased, therefore micronization (particle size reduction) may lead to success in those cases. Other compounds will never dissolve no matter how small the particle size is. An increasing percentage of new chemical entities (NCEs) exhibit poor aqueous solubility which results in poor bioavailability and those properties cannot be improved with traditional solid-state techniques (salt selection, cocrystal development, micronization). These molecules may be suited for development of an amorphous dispersion by spray drying, hot melt extrusion, or other methods.Triclinic offers development and formulation services to guide amorphous material development (spray-drying, hot-melt extrusion, cryogrinding, particle engineering). We can determine stability of materials produced in a variety of ways (e.g. varying formulations), and can develop validated assays to determine if the material is amorphous or contains crystalline components. We are the listed lab in innumerable FDA filings for cGMP solid dispersion testing and release methods. To schedule a discussion of for more information on amorphous material development, please click here.
To learn more about our amorphous material formulation development approaches including spray drying and holt melt extrusion, click here.
Gain insight into the stability and structure of Non-Crystalline (X-ray Amorphous) Systems and Solid Dispersions:
A non-crystalline material (amorphous solid) is one where the measured X-ray powder diffraction (XRPD) pattern is essentially continuous in appearance. These types of powder patterns are often referred to as "X-ray amorphous". The characterization of non-crystalline materials is a further application of the Total Diffraction Analysis services offered by the Computational Chemistry Practice at Triclinic. Through the application of in-house analytical software based upon the Debye diffraction theory (atom-atom pair correlation), the local amorphous structure can be elucidated from an X-ray amorphous powder pattern.
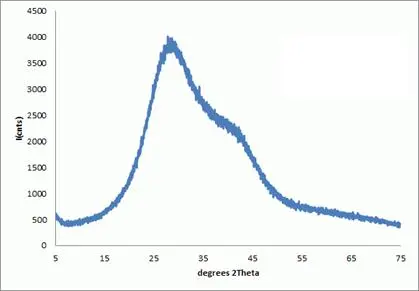
Figure 1: A continuous X-ray diffraction pattern for water; measured in-house. Liquids are thermodynamically stable non-crystalline forms.
Structural analysis of non-crystalline materials.
An ideal gas, ideal liquid, and ideal glass all represent the same highest symmetry state for a molecular system and when averaged over a suitable time period and spatial volume, the probability of finding a molecule at any point in space is a constant related to density. These high symmetry states have the full translation and rotation symmetry of free space and full conformation degrees of freedom appropriate for the system temperature. These systems are considered to be macroscopically uniform and isotropic. Any effective local molecular order will involve single molecules and will be related to just the rigid intra-molecular structure itself. In reality, the high density and high viscosity of a glassy system will force the formation of locally rigid and high density arrangements of molecules where the nearest neighbor positional relationships will be driven the repulsive inter molecular forces (i.e. molecular shape). With respect to the locally ordered groups, the full translation and rotation symmetry of free space is maintained keeping the macroscopically uniform nature of a glass. It is these locally rigid arrangements of molecules that give rise to the observed X-ray amorphous powder patterns.Glassy materials are just one example of solid state amorphous systems that will give rise to X-ray amorphous powder patterns. Any single-phase non-crystalline material with reproducible short-range molecular order and no long-range molecular order will give rise to an X-ray amorphous powder pattern. Characterization of the local molecular order is a fundamental component in understanding the chemical and physical stability of non-crystalline materials.
Total Diffraction Analysis as a structural analysis tool.
Total diffraction analysis is one of the main characterization methods for determining the local structure within non-crystalline materials (amorphous solids). It makes use of the complete diffraction signal from a sample and treats each data point as an individual observation. This allows the subtle changes in slope that define a continuous powder pattern to be directly incorporated into the analysis. A number of general material properties can be determined via direct analysis of the X-ray amorphous powder pattern. Because of the nature of random packing in the solid state, a randomly packed material will give X-ray amorphous halos whose width follows a known functional form as a function of diffraction angle. As such, a quick determination of the observed halo widths can be used to determine the departure from random packing for a particular sample. A detailed structural analysis, however, requires the use of novel analytical methods based upon the Debye diffraction theory and the use of a continuous structure factor. The Debye approach can be applied directly through the use of Pair-wise Distribution Functions (PDF) or indirectly through molecular modeling.
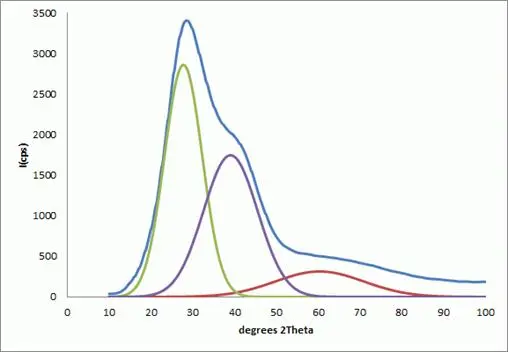
Figure 2: Specific halos observed in the continuous X-ray diffraction for water can be modeled according to the universal peak width idea for randomly packed materials. For this data the exponent describing random packing came out to be 4.93; a randomly packed material has N~5 as determined by XRPD.
Amorphous Materials have Local Molecular Order
Characterization of a significant number of non-crystalline materials has shown that different types of local molecular order can exist for organic molecular systems. In more than half of the studied materials, the X-ray amorphous powder pattern can be described using a local molecular model that matches structural motifs seen in one or more of the crystalline polymorphs. As might be expected, these types of X-ray amorphous forms appear to crystallize readily. The remaining non-crystalline materials gave X-ray amorphous powder patterns that can be described using local molecular models that maximize density and minimize the short range repulsive forces. For approximately flat 2D molecules, this usually results in stacking of the molecule. In some instances the type of local order observed can be modified by adopting a different production path way.Changes in the local structure observed as a function of time, temperature, humidity, solvent vapor or physical stress can often been seen as precursors to structural instability and re-crystallization. Characterization of local structure changes can be critical to the design of an effective aging study as very often the humidity and temperature conditions used for aging actually change the local structure before the study begins.
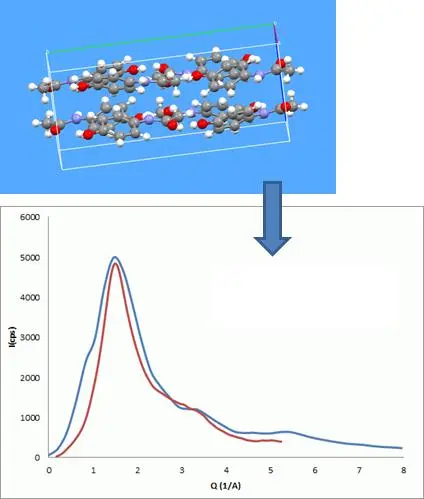
Figure 3 : A calculated X-ray diffraction trace (blue) for a glassy material having the local molecular order corresponding to Form-II acetaminophen crystal structure (image above) gives a reasonable description of the measured XRPD trace for glassy acetaminophen (red).
While Total Diffraction Analysis provides the initial characterization of local molecular order, for some systems the changes in the X-ray amorphous profile can be subtle and a more robust analysis will benefit from a combination of different analytical techniques (for example IR/Raman spectroscopy, thermal methods and X-ray powder diffraction).
Predicting and Controlling Amorphous Stability
A common method to improve the physical stability of an amorphous API is to manufacture a ‘dispersion’ of the amorphous API in an amorphous matrix. The most common matrix materials employed to date are polymers with high glass transition temperatures although in principal many different types of matrix materials may be used. The primary physical property that determines the performance ability of the amorphous dispersion to stabilize the amorphous state of the API is the degree of mixing between the API and the amorphous matrix. In the liquid or solution state the API and matrix components may be intimately mixed at the molecular level but on formation of the solid state system, the API may begin to phase separate. The degree to which the phase separation has occurred in the solid state will govern the resulting physical stability of the amorphous ‘dispersion’.Systems where the API can be considered to be phase separated will behave like the pure amorphous API but will often undergo physical changes more slowly. Systems where the API and matrix remain a solid solution with intimate molecular mixing may form a stable single phase system with no driving force to crystallize. The concepts of molecular solid solutions and phase separated systems are defined by the physical structure and spatial relationships of the molecules within the solid-state. These are exactly the properties measured by X-ray (and neutron) diffraction. The same in-house software Triclinic has developed to characterize the local molecular order of single phase X-ray amorphous materials can be utilized to determine the degree of mixing between an amorphous API and an amorphous carrier matrix.
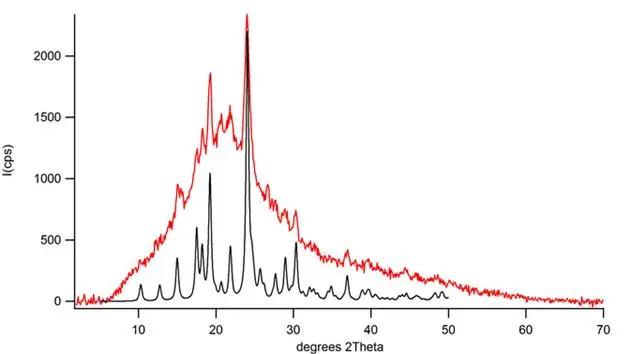
Figure 4: Glassy acetaminophen which gives an X-ray diffraction trace similar to the calculated trace from a theoretical glassy Form-II sample, is seen to readily re-crystallize to Form-II.
Total Diffraction Analysis is a structural analysis tool:
- Returns ‘structure’ of the amorphous phase.
- Considers density of amorphous forms - typically within a few percent of crystalline forms. True random packed materials have significantly lower densities than crystalline materials.
- There is considerable short range order in molecular organic amorphous materials - often very similar in nature to crystalline order.
- Makes use of all the diffraction signal from the sample
- Every data point is an observation
-
Requires different analytical tools than traditional Bragg diffraction analysis (peak based):
- Debye diffraction theory
- Requires building molecular models
- Pair Wise Distribution Function (PDF)
- Continuous structure factor based
Use of Multiple Analytical Techniques for Amorphous Material Analysis
MDSC measurements of glass transition temperatures will also give some idea as to the degree of mixing between an amorphous API and an amorphous matrix. Typically, if the observed glass transition temperature of the amorphous ‘dispersion’ (assuming one can be measured) is between the glass transition temperatures of the pure amorphous API and pure amorphous matrix then some degree of molecular intermixing is taking place. Although for some dispersion systems showing a single intermediate glass transition temperature characterized by Total Diffraction Analysis as actually being phase separated and not molecularly dispersed. It is believed that for these types of systems, the domain sizes of the separated phases are very small (~<30nm). For robust characterization of amorphous materials, a combined analytical approach using spectroscopic, thermal and diffraction methods is the preferred approach. With recent advances in characterization software, considerably more information can now be extracted from amorphous materials using diffraction techniques than was previously possible. Characterization of the local molecular short-range order using X-ray powder diffraction data provides unique insight into single phase and multi component amorphous systems that is often directly related to the physical and chemical stability of the amorphous system.Recent Applications of Total Diffraction and Amorphous ‘Structure’ Determination
While the determination of the local structure in amorphous materials may seem to be a topic of mainly academic interest, general examples taken from recent client projects at Triclinic Labs illustrate the significant impact this type of analysis can have on real world production problems.-
The first example involves an API production process where the client was experiencing flow problems for
certain batches. Traditional analytical chemistry methods using the traditional tools (Thermal, Structural
and Vibrational analysis) were unable to find any correlation between the flow problem and the analytical
responses from samples taken from the various API batches. Total Diffraction Analysis, however, revealed
the presence of a mesomorphic component in some batches of the material and that the flow problem
correlated well with the concentration of this component.
- A second example centers on a Hot Melt Extrusion drug product where an increase in physical instability was noticed for some batches of the material. A combination of Total Diffraction Analysis with a chemometric method developed at Triclinic labs was able to identify the degree of API phase separation for each batch and thus the degree of risk associated with the different drug product batches.
- A third example represents a study of different lyophilization procedures in order to characterize the ratio of free to bound water. Total Diffraction Analysis is more than capable of quantifying the amount of phase separated water within a lyophile sample.
Why Triclinic?
Triclinic has more than two decades of experience in finding new solid forms, evaluating their physico-chemical properties, and selecting the best form for commercialization. We've collaborated with companies large and small on thousands of polymorph, salt, amorphous material, and cocrystal projects.- The scientists at Triclinic have performed thousands of polymorph and salt screens and more the 300 cocrystal screens to date.
- We have extensive experience in amorphous material development and formulation. We have proprietary methods for determining various stabilities of formulations.
- We specialize in solid form development and have industry leading experience in discovering and developing solid forms for chemical and intellectual property improvement.
- Our cocrystal hit rate is extremely high (75% of the 100 APIs we screened in the last 12 months resulted in at least one cocrystal)
- We have industry leading proven proprietary screening techniques that are only available at Triclinic. We constantly update and improve our screening approaches.
- Our database of pharmaceutically acceptable coformers is more diverse and better researched than any other (more than 300 in 2022)
What's it cost and how long does it take?
Ahhh - the magic (and usually final) questions! The cost and time requirements for screening depend on the number of experiments and the number of usable samples analyzed. Scale up and characterization of a solid form or amorphous material development found during screening adds additional cost and time. Without knowing how many solid forms will be found, it is difficult to estimate total project cost. But we know you need those numbers. So we usually have a short discussion with you and build a customized proposal to meet your time and project goals. There's no cost involved and we can usually generate a quote within a few days. So please contact us:
Novel Non-Crystalline Materials Analysis: New Strategies to De-risk Amorphous Material Formulation Development.
Simon Bates, Ph.D.The latest approaches for characterizing non-crystalline (amorphous) materials and determining physical stability under typical storage conditions.
Amorphous forms consist of disordered arrangements of molecules that do not possess a distinguishable
crystal lattice.
Different forms of a drug substance can have different chemical and physical properties, including melting
point, chemical reactivity, apparent solubility, dissolution rate, optical and mechanical properties,
vapor pressure, and density. These properties can have a direct effect on
the ability to process and/or manufacture the drug substance and the drug product, as well as on
drug product stability, dissolution, and bioavailability. Instability of non-crystalline materials is of
particular concern to regulatory bodies within companies and national agencies.
Download The Complete Tech Note here:

Utility of Low Frequency (LF) Raman Mapping: Dissolution of Acetaminophen (ACE) Spray Dried Dispersions
(SDD)
Nico Setiawan, Ph.D., Andrew Smith, and David E. Bugay, Ph.D.
This application note outlines the evaluation of post-dissolution ACE SDD. The results indicated that in all of the cases studied, ACE remained amorphous upon dissolution if it was intimately mixed in the dispersion. However, phase separation from the polymer allowed ACE to crystallize as expected. Interestingly, the type of polymer used affected the ACE polymorph generated. The mixture of polymorphs was observed in the powder X-ray diffraction (PXRD) result (though it could easily be overlooked), while LF Raman maps showed distinct regions of the different polymorphs. This study demonstrated the utility of LF Raman mapping in elucidating the dissolution mechanism of amorphous dispersions.
Keywords: Low Frequency Raman, Amorphous, Spray dried dispersion, Dissolution, Crystallization, Polymorphs
